UK GUIDELINES ON TRANSPLANTATION FROM DECEASED DONORS AFTER CIRCULATORY DEATH
Chapters:
2. Executive summary of recommendations
3. Categorisation of DCD donors and definition of warm ischaemic time
5. Law, ethics and donor consent
7. Normothermic regional perfusion
9. Kidney
10. Liver
11. Pancreas
12. Islets
13. Lung
14. Heart
Introduction and the Need for Guidelines
Transplantation offers patients with end-stage organ failure a cost-effective treatment that can improve quality of life and increase life expectancy. Prior to the introduction of guidance defining the concept of brain death in the 1970s, all organs donated for transplantation were from donation after circulatory death (DCD) donors. Following the introduction of neurological testing to diagnose death, the majority of organs donated for transplantation were from donation after brain death (DBD) donors or living donors.
In 1999/2000 there were 33 DCD donors in the United Kingdom (UK) (1). By 2011/12 the number of DCD donors in the UK had increased steadily to 436 before rising to a peak of 639 in 2018/19, before falling during the coronavirus disease 2019 (COVID-19) pandemic. Despite this progress, organ demand continues to outstrip availability. By March 2021, 4,256 people in the UK remained on the waiting list for an organ transplant, with 474 patients dying in the preceding year while active on the list (an increase of 26% from the previous year) (2).
An average of 2.7 organs are currently donated per DCD donor compared to 3.3 organs per DBD donor. In 2020/21, 52% of DCD donors were donors of a single organ type, with the majority (96%) of these donors donating just their kidneys (2). Further maximising organ utilisation from DCD donors could help meet some of the shortfall in organ supply, but it represents a major challenge, particularly as organ donors and transplant recipients become older and more medically complex over time (3).
‘Uncontrolled’ DCD donation refers to organ donation after failed efforts to resuscitate an individual experiencing an out-of-hospital or unexpected in-hospital cardiopulmonary arrest. ‘Controlled’ DCD donation refers to organ donation from donors who have died in hospital following the withdrawal of life-sustaining treatment (WLST) (4). Despite some historical success with uncontrolled DCD kidney programmes in the UK, all organs in the UK donated from DCD donors are currently in a controlled (Maastricht category III or IV) setting (5, 6). This Guideline will focus exclusively on controlled DCD donation (Chapter 3).
The British Transplantation Society (BTS) first published a Guideline for transplantation from DCD donors in 2004 under the title ‘Guideline relating to solid organ transplants from non-heart-beating donors’ (7). The Guideline was updated in 2013 under the title ‘Guideline on transplantation from deceased donors after circulatory death’ (8). Relevant updates to policies and guidance from NHS Blood and Transplant (NHSBT) are also available online (https://www.odt.nhs.uk/transplantation/tools-policies-and-guidance/policies-and-guidance) and can be read in conjunction with these Guidelines.
This document extends and updates the previous Guidelines and aims to include recent developments in all aspects of DCD clinical practice for all relevant organs. Several significant developments have occurred in the intervening period between this Guideline and the previous versions, including the increasing use of novel machine perfusion technology (9), the introduction of opt-out legislation across the UK (10), progress in DCD heart transplantation (11), the potential for utilising organs from paediatric DCD donors (12), the use of DCD organs for transplantation in paediatric recipients (13), and the risks presented by the COVID-19 pandemic.
Recipient outcomes after transplantation with organs from DCD donors can compare favourably and even match recipient outcomes after transplantation with organs from DBD donors (11, 14-17). Success is dependent upon establishing common practices and accepted protocols that allow the safe sharing of DCD organs and maximise the use of the DCD donor pool. Optimal donor management and careful recipient selection are pivotal to facilitating the donation of as many organs as possible, and it is essential that organ offering systems account for recipient needs and organ utilisation to maximise transplant benefit. It is hoped that these Guidelines will harmonise practice and set the direction for further expansion of DCD organ donation and transplantation in the UK and beyond.
Process of writing and methodology
In April 2021, the Chair and Vice Chair of the BTS Standards and Guidelines committee, Dr Ellie Asgari and Mr Chris Callaghan, emailed a request to BTS members for expressions of interest to work on an update of the BTS Guidelines on transplantation from deceased donors after circulatory death (8).
Following a review of the expressions of interest, a Guideline development group was formed in May 2021, co-chaired by Mr Stephen O’Neill and Mr David Nasralla. Virtual meetings were held in June 2021 and July 2021 with Dr Ellie Asgari, Mr Chris Callaghan, Mr Stephen O’Neill and Mr David Nasralla to establish the scope of the Guideline and the additional required individual contributors who would be approached to work on each chapter.
The Guideline was written in line with the BTS Guideline Development Policy, and the recommendations of NICE Evidence (18). Each group of contributors performed their own literature search using PubMed® to identify relevant evidence. Virtual progress meetings between the Guideline development group and contributors were held in September 2021, November 2021, January 2022 and June 2022. A review of draft chapters and preliminary grading of recommendations was then conducted in July and August 2022. A face-to-face meeting was then held in Belfast in September 2022 for review and discussion of the final grading of the recommendations.
Comments on the preliminary draft were invited from patient representatives. The Guidelines were edited by Dr Ellie Asgari and Mr Chris Callaghan and were opened for public consultation through the website of the BTS in May 2023. The received comments were reviewed and actioned, resulting in the current guideline version.
Contributing Authors
Dr Miriam Berry, Consultant Nephrologist, Queen Elizabeth Hospital, Birmingham
Mr Marius Berman, Consultant Cardiac Surgeon, Royal Papworth Hospital, Cambridge
Professor Paul Corris, Emeritus Professor of Thoracic Medicine, Newcastle University and Institute of Transplantation, Freeman Hospital, Newcastle
Miss Miriam Cortes Cerisuelo, Consultant Transplant Surgeon, King’s College Hospital, London
Professor Heather Draper, Professor of Ethics, Warwick University
Dr Peter Dupont, Consultant Nephrologist, Royal Free Hospital, London
Dr Dale Gardiner, Consultant Intensivist, Queen’s Medical Centre, Nottingham
Mrs Patty Gilbert, Northern Ireland Kidney Patients’ Association, Belfast
Miss Hermien Hartog, Consultant Transplant Surgeon, Queen Elizabeth Hospital, Birmingham
Mr Satheesh Iype, Consultant Transplant Surgeon, Royal Free Hospital, London
Professor Paul Johnson, Consultant Paediatric Surgeon, John Radcliffe Hospital, Oxford
Mr Nikolaos Karydis, Consultant Transplant Surgeon, Guy’s Hospital, London
Mr Stephen Large, Consultant Cardiac Surgeon, Royal Papworth Hospital, Cambridge
Dr Alex Manara, Consultant Intensivist, Southmead Hospital, Bristol
Mr Simon Messer, Consultant Cardiac Surgeon, Golden Jubilee Hospital, Glasgow
Ms Jean Michelo, Northern Ireland Kidney Patients’ Association, Belfast
Dr Greg Moorlock, Associate Professor of Ethics, Warwick Medical School
Mr David Nasralla, Consultant Transplant Surgeon, Royal Free Hospital, London
Miss Anisu Nutu, Transplant Fellow, Queen Elizabeth Hospital, Birmingham
Mr Stephen O’Neill, Consultant Transplant Surgeon, Belfast City Hospital, Belfast
Mr Gabi Oniscu, Consultant Transplant Surgeon, Royal Infirmary, Edinburgh
Dr Jas Parmar, Consultant Respiratory Physician, Royal Papworth Hospital, Cambridge
Mr Thamara Perera, Consultant Transplant Surgeon, Queen Elizabeth Hospital, Birmingham
Mr Benedict Phillips, Specialty Registrar in Transplant Surgery, Guy’s Hospital, London
Mr Sanjay Sinha, Consultant Transplant Surgeon, Churchill Hospital, Oxford
Mr Andrew Sutherland, Consultant Transplant Surgeon, Royal Infirmary, Edinburgh
Mr David Van Dellen, Consultant Transplant Surgeon, Manchester Royal Infirmary, Manchester
Mr Peter Veitch, Consultant Transplant Surgeon, Belfast City Hospital, Belfast
Professor Chris Watson, Consultant Transplant Surgeon, Addenbrooke’s Hospital, Cambridge
Professor Steve White, Consultant Transplant Surgeon, Freeman Hospital, Newcastle
Mr Vasudev Pai, Consultant Cardiac Surgeon, Royal Papworth Hospital, Cambridge
Conflicts of Interest
No conflicts of interest were declared.
Grading of Recommendations
These Guidelines represent consensus opinion from experts in the field of transplantation in the UK. They represent a snapshot of evidence available at the time of writing. It is recognised that in some areas, recommendations are made even when the evidence is weak. It is felt that this is helpful to clinicians in daily practice.
In these Guidelines the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system has been used to rate the strength of evidence and the strength of recommendations (19). The approach used in producing the present Guidelines is consistent with that adopted by Kidney Disease Improving Global Outcomes (KDIGO) (20, 21). Explicit recommendations are made on the basis of the trade-offs between the benefits on one hand, and the risks, burden, and costs on the other.
For each recommendation the quality of evidence has been graded as:
A (high)
B (moderate)
C (low)
D (very low)
Grade A evidence means high quality evidence that comes from consistent results from well-performed randomised controlled trials, or overwhelming evidence of another sort (such as well-executed observational studies with very strong effects).
Grade B evidence means moderate quality evidence from randomised trials that suffer from serious flaws in conduct, consistency, indirectness, imprecise estimates, reporting bias, or some combination of these limitations, or from other study designs with special strength.
Grade C evidence means low quality evidence from observational evidence, or from controlled trials with several very serious limitations.
Grade D evidence is based only on case studies or expert opinion.
For each recommendation, the strength of recommendation has been indicated as one of:
Level 1 (we recommend)
Level 2 (we suggest)
Not graded (where there is not enough evidence to allow formal grading)
A Level 1 recommendation is a strong recommendation to do (or not to do) something where the benefits clearly outweigh the risks (or vice versa) for most, if not all patients.
A Level 2 recommendation is a weaker recommendation, where the risks and benefits are more closely balanced or are more uncertain.
Abbreviations
ALT Alanine transaminase
AKI Acute kidney injury
BTS British Transplantation Society
CNI Calcineurin inhibitors
COVID-19 Coronavirus disease 2019
CPR Cardiopulmonary resuscitation
DBD Donation after brain death
DCD Donation after circulatory death
DGF Delayed graft function
ECD Extended criteria donor
ECMO Extracorporeal membrane oxygenation
EVLP Ex-vivo lung perfusion
FWIT Functional warm ischaemia time
GFR Glomerular filtration rate
GRADE Grading of Recommendations Assessment, Development and Evaluation
HAT Hepatic artery thrombosis
IC Ischaemic cholangiopathy
KDIGO Kidney Disease Improving Global Outcomes
NHBD Non-heart beating donation
NRP Normothermic regional perfusion
OCS Organ Care System
PA Pulmonary artery
PNF Primary non-function
SCD Standard criteria donor
SNOD Specialist Nurse in Organ Donation
TA-NRP Thoraco-abdominal normothermic regional perfusion
TESMP Total time of ex situ machine perfusion
UK United Kingdom
WLST Withdrawal of life-sustaining treatments
Definitions and Scope
This Guideline covers the categorisation of DCD donors, definitions of warm ischaemic time, diagnosis of death, law, ethics, donor consent, organ retrieval, normothermic regional perfusion, informing the recipient, kidney transplantation, liver transplantation, pancreas transplantation, islet cell transplantation, lung transplantation and heart transplantation. Transplantation in adult and paediatric recipients is considered.
Disclaimer
This document provides a guide to best practice, which inevitably evolves over time. All clinicians involved in these aspects of transplantation need to undertake clinical care on an individualised basis and keep up to date with changes in the practice of clinical medicine.
This Guideline represents the collective opinions of experts in the field and do not have the force of law. They contain information/guidance for use by practitioners as a good practice tool. It follows that the Guideline should be interpreted in the spirit rather than the letter of their contents. The opinions presented are subject to change and should not be used in isolation to define the management of any individual patient.
The Guideline is not designed to be prescriptive, nor to define a standard of care. The BTS cannot attest to the accuracy, completeness or currency of the opinions contained herein and does not accept responsibility or liability for any loss or damage caused to any practitioner or any third party as a result of any reliance being placed on the guideline or as a result of any inaccurate or misleading opinion contained in the guideline.
References
- Summers DM, Counter C, Johnson RJ, et al. Is the increase in DCD organ donors in the United Kingdom contributing to a decline in DBD donors? Transplantation 2010;90(12):1506-10.
- Organ and Tissue Donation and Transplantation Activity Report 2020/21. https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/24053/activity-report-2020-2021.pdf.
- Organ Donation and Transplantation 2030: Meeting the Need. https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/23463/meeting-the-need-2030.pdf.
- Domínguez-Gil B, Ascher N, Capron AM, et al. Expanding controlled donation after the circulatory determination of death: statement from an international collaborative. Intensive Care Med 2021 Mar;47(3):265-81.
- Koostra G, Daemen JH, Oomen AP. Categories of non-heart-beating donors. Transplant Proc 1995;27(5):2893-4.
- Thuong M, Ruiz A, Evrard P, et al. New classification of donation after circulatory death donors definitions and terminology. Transpl Int 2016;29(7):749-59.
- British Transplantation Society Guidelines relating to solid organ transplants from non- heart beating donors, January 2004.
- British Transplantation Society Guidelines on Transplantation from deceased donors after circulatory death. https://staging-d.bts.org.uk/wp-content/uploads/2016/09/15_BTS_Donors_DCD-1.pdf .
- O’Neill S, Srinivasa S, Callaghan CJ, et al. Novel organ perfusion and preservation strategies in transplantation – where are we going in the United Kingdom? Transplantation 2020;104(9):1813-24.
- Noyes J, McLaughlin L, Morgan K, et al. Short-term impact of introducing a soft opt-out organ donation system in Wales: before and after study. BMJ Open 2019 Apr 3;9(4):e025159.
- Messer S, Cernic S, Page A, et al. A 5-year single-centre early experience of heart transplantation from donation after circulatory-determined death donors. J Heart Lung Transplant 2020 Dec;39(12):1463-75.
- Ghavam A, Thompson NE, Lee J. Comparison of pediatric brain-dead donors to donation after circulatory death donors in the United States. Pediatr Transplant 2021 May;25(3):e13926.
- Marlais M, Pankhurst L, Hudson A, et al. UK national registry study of kidney donation after circulatory death for pediatric recipients. Transplantation 2017 Jun;101(6):1177-81.
- Callaghan CJ, Ibrahim M, Counter C, et al. Outcomes after simultaneous pancreas-kidney transplantation from donation after circulatory death donors: A UK registry analysis. Am J Transplant 2021 Nov;21(11):3673-83.
- Summers DM, Watson CJ, Pettigrew GJ, et al. Kidney donation after circulatory death (DCD): state of the art. Kidney Int 2015 Aug;88(2):241-9.
- Ruiz P, Valdivieso A, Palomares I, et al. Similar results in liver transplantation from controlled donation after circulatory death donors with normothermic regional perfusion and donation after brain death donors: a case-matched single-center study. Liver Transpl 2021 Dec;27(12):1747-57.
- Van Raemdonck D, Keshavjee S, Levvey B, et al. International Society for Heart and Lung Transplantation. Donation after circulatory death in lung transplantation-five-year follow-up from ISHLT Registry. J Heart Lung Transplant 2019 Dec;38(12):1235-45.
- Andrews PA. BTS Guideline Development Policy 2016. Accessed at https://staging-d.bts.org.uk/wp-content/uploads/2017/06/11_BTS_Guideline_Development_Policy_2-1-1.pdf.
- Atkins D, Best D, Briss PA, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. Br Med J 2004;328:1490.
- Uhlig K, Macleod A, Craig J, et al. Grading evidence and recommendations for clinical practice guidelines in nephrology. A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2006;70:2058-65.
- Kidney Disease Improving Global Outcomes (KDIGO) Transplant Work Group: KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009;9(S3):S1-157.
2. Executive Summary of Recommendations
Recommendations should be read in conjunction with the accompanying chapter.
Chapter 3 Categorisation of DCD Donors and Definition of Warm Ischaemic Time
We recommend that:
- Deceased circulatory death (DCD) donors should be categorised according to the Maastricht classification to aid research, communication and audit. (1B)
- The functional warm ischaemia time starts when the systolic blood pressure has a sustained (i.e., at least two minutes) fall below 50 mmHg and extends up to the onset of cold in situ perfusion or normothermic regional perfusion. (1B)
Chapter 4 Diagnosis of Death
We recommend that:
- The decision to withdraw life-sustaining treatment and criteria for the diagnosis of death must not be influenced by the possibility of subsequent organ recovery. (1A)
- The process of controlled DCD must be consistent with the provision of high-quality end-of-life care for a dying patient and their family. (1A)
- Death is defined as the permanent loss of the capacity for consciousness and loss of all brainstem functions. This may result from the permanent cessation of circulation and/or after catastrophic brain injury. Death is confirmed using circulatory criteria in the former and neurological criteria in the latter. (1A)
- In the context of death determination, ‘permanent’ refers to loss of function that cannot resume spontaneously and will not be restored through intervention. (1A)
- Where circulatory criteria are used, death can be confirmed after five minutes of continuous cardio-respiratory arrest, providing there is no subsequent intervention with the potential to restore cerebral perfusion. (1A)
- Where possible, circulatory arrest should be identified and monitored by the absence of pulsatile flow on a correctly functioning arterial line or using echocardiography if the expertise is available; or failing that, by continuous ECG monitoring. (1C)
- DCD organ recovery protocols should recognise the potential risks around post-mortem interventions that might restore cerebral perfusion and implement strategies to prevent this. (1C)
Chapter 5 Law, Ethics and Donor Consent
We recommend that:
- ‘Overall benefit’ should be the guiding principle when making decisions about end-of-life care in relation to organ donation. (1D)
- The strength of a patient’s wishes and decisions regarding organ donation should be included in any consideration of overall benefit. (1D)
- It should not be assumed that someone opting-in to organ donation necessarily has a stronger wish to donate their organs than someone who did not record a preference. (1D)
- Appropriate frameworks/guidance (‘Donation Actions Framework’ in England, Wales and Northern Ireland; ‘Guidance on the authorisation and undertaking of pre-death procedures’ in Scotland) should be used to guide decision-making regarding what actions are ethically and legally permissible in the context of DCD. (1D)
- Further consideration is given to ethical considerations related to undertaking organ donation research, particularly in relation to consent for such activities. (1D)
Chapter 6 Organ Retrieval
We recommend that:
- Treatment withdrawal should ideally be planned for a time when the donor HLA type and virology are known and the thoracic, liver and pancreas organ offers have been accepted or declined by recipient centres. (1C)
- Treatment withdrawal in the operating department is associated with a shorter asystolic warm withdrawal on a remote intensive care unit. The logistics to achieve a shorter asystolic warm period after confirmation of death should be discussed by the NORS team with the SNOD. (1C)
- The retrieval team need to be satisfied about the donor details (blood group, past medical history, illness leading to death etc.) before treatment is withdrawn. (1A)
- Retrieval teams should be prepared in the operating theatre at the point of treatment withdrawal. (1B)
- In Maastricht 4 donors, where death has been established previously by neurological criteria, heparin (or other appropriate treatments) may be given just prior to treatment withdrawal. (1A)
- In Maastricht 4 donors, where death has been established previously by neurological criteria, death does not need to be reaffirmed once circulatory arrest has occurred, but five minutes of continuous cardio-respiratory arrest should still be observed. (1A)
- For controlled donors, retrieval starts by gaining access to a large artery and vein, typically the right common iliac artery or aorta, and the IVC in the abdomen or right atrium in the chest. (1B)
- Cannulae for infusion of preservation fluid should not be placed in the SMV or IMV when the pancreas is being retrieved. (1B)
We suggest that:
- There is no substantive evidence for adding a fibrinolytic agent such as streptokinase or recombinant tissue plasminogen activator to the preservation solution. (2B)
- Heparin should be added to the first two litres of preservation solution to be perfused through the aorta and the portal vein at a dose of 300 IU/kg donor bodyweight (around 25,000 IU for an 80kg person). (2B)
- The liver should be recovered using a rapid technique that minimises liver congestion. (2C)
- Dual perfusion of the hepatic artery and portal vein is preferred for recovery of DCD livers before transplantation, apart from in infant donors of <15kg body weight. (2C)
- In larger donors, the kidneys may be removed either individually or en bloc. (2D)
- The pancreas may be removed either en bloc with the liver, or separately from the liver. (2D)
Chapter 7 In situ Normothermic Regional Perfusion
We recommend that:
- Normothermic regional perfusion (NRP) should be used in all DCD retrievals where there is a trained team competent to perform it. (1B)
- If the ALT after two hours of NRP is under 500 iu/L and there are otherwise no contraindications, the liver should be considered suitable for transplantation. Beyond that, further ex situ assessment may be necessary. (1C)
We suggest that:
- Femoral cannulation is preferred if a thoracic retrieval team is present. (2C)
- The duration of the withdrawal period is not viewed as a contraindication to utilising a liver from a DCD donor that is recovered using NRP. (2C)
Chapter 8 Informing the Recipient
We recommend that:
- Providing information, both orally and in writing, for the potential transplant recipient is a requirement for consent and is the responsibility of the multi-disciplinary transplant team. This must be updated and reviewed annually, and the outcome of discussions to be clearly documented in the patient’s medical record. (1B)
- Information should be tailored to the requirements of the potential recipient, recognising that not all patients wish to receive detailed information. However, this must not preclude engagement with the transplant process. (1B)
- The final risk-benefit analysis presented to the potential transplant recipient following an organ offer must explain the relative risk for that recipient of remaining on the transplant waiting list compared to that of receiving a DCD organ. (1B)
We suggest that:
- Final consent for transplantation of a DCD organ, where the donor type has a significant impact on expected organ outcomes, should not normally be delegated, particularly given the complexities around the discussion of alternative strategies, like waiting for another organ offer. (2D)
- Units generate consent addenda that adhere to NHSBT/BTS guidance covering risks and benefits specific to transplantation of different organ types where the donor type has a significant impact on expected organ outcomes that should include information regarding transplantation of organs from DCD donors. (2D)
Chapter 9 Kidney
We recommend that:
- The incidence of delayed graft function should be discussed with the patient prior to transplantation. (1A)
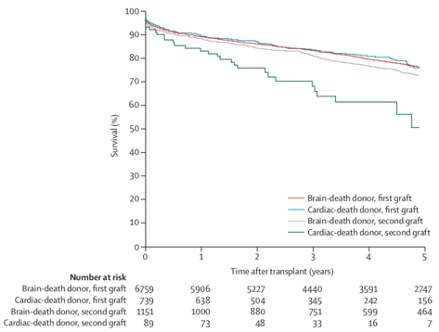
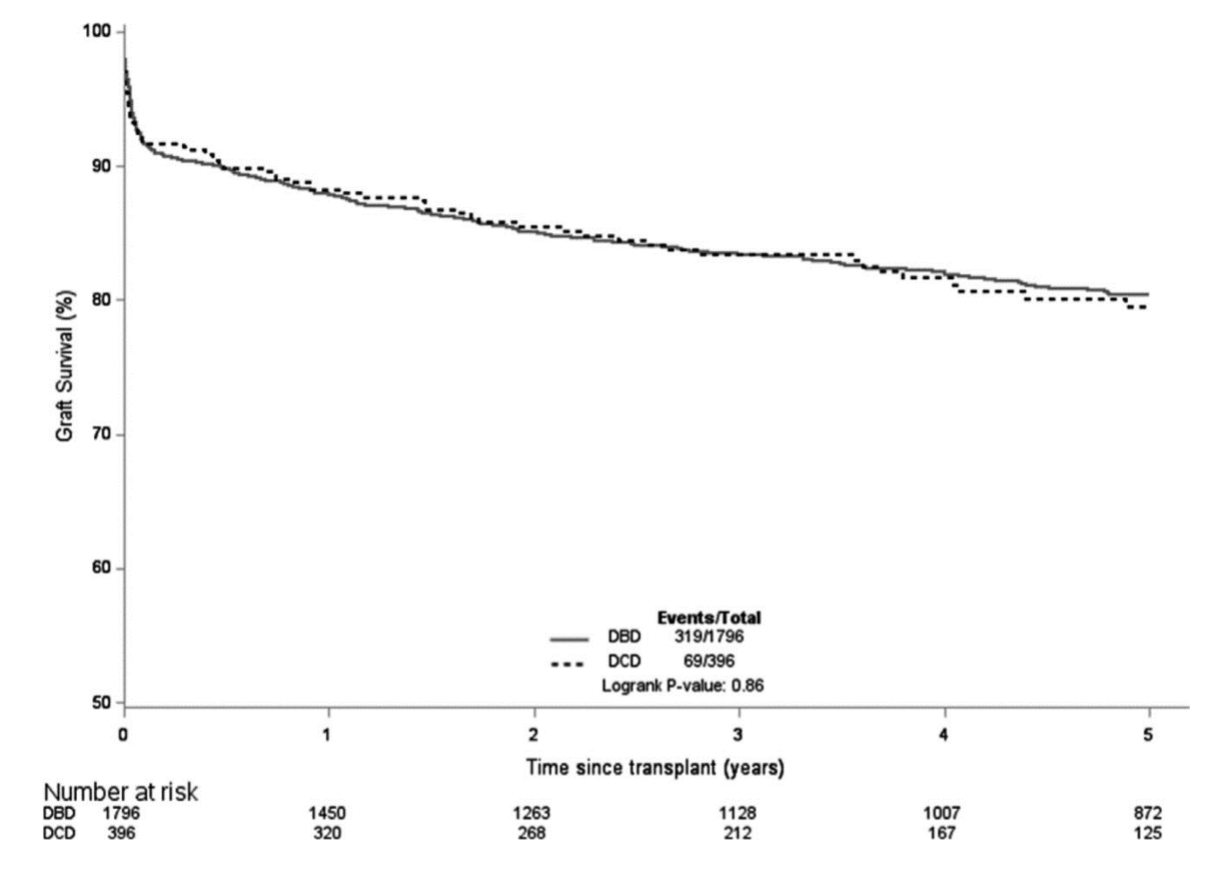
- Potential recipients should be informed that long-term outcomes for standard criteria donors are equivalent for DCD and DBD kidney transplants. (1A)
- Contraindications to kidney donation do not differ according to deceased donor type. (1B)
- As the long-term outcomes of DCD recipients are similar to those of DBD recipients, the organ offering system for DCD and DBD donor organs should be similar. Nevertheless, it is recognised that DCD donor kidneys are more susceptible to cold ischaemia than DBD kidneys and should be implanted with cold ischaemic time of <12 hours, where possible. (1B)
- There is no evidence to support the use of alternative immunosuppression strategies in DCD donor kidney transplants beyond the standard of care. (1B)
We suggest that:
- None of the hypothermic machine perfusion perfusate effluent biochemical analysis or perfusion pressure dynamic characteristics, or kidney transplant biopsy scoring systems – either alone or in combination – have sufficient predictive value to mandate organ discard. (2A)
- Cold machine perfusion may reduce the incidence of delayed graft function in recipients of DCD donor kidneys when performed from the point of retrieval. (2B)
- The presence of donor AKI in DCD kidneys be considered in the context of individual patient factors. (2B)
- The use of kidneys from donors with a withdrawal time >3 hours or absent blood pressure for >30 minutes should be restricted to protocols that attempt to resuscitate organ viability. (2C)
- Normothermic regional perfusion may reduce the incidence of delayed graft function and improve kidney function in DCD kidneys. (2B)
- In paediatric recipients, the rate of DGF and PNF is higher in DCD donor kidneys compared to DBD donor kidneys. However, three-year graft survival is comparable. (2B)
Chapter 10 Liver
We recommend that:
- All centres should be prepared to use livers from DCD donors for transplantation. (1B)
- The outcome of transplanting DCD livers recovered without normothermic regional perfusion is improved with short CIT, and CIT is recommended to be kept under 8 hours. (1B)
- Ex situ preservation time can be extended beyond 8 hours when in situ normothermic regional perfusion has been used. (1B)
- Ex situ preservation time can be extended beyond 8 hours when ex situ machine perfusion is used, but the impact on ischaemic cholangiopathy is unknown. (1B)
- If the functional warm ischaemia time exceeds 30 min and organs are not being recovered using in situnormothermic regional perfusion or hypothermic machine perfusion is not being used, there is an increased risk for graft loss, however further donor and recipient characteristics should be taken into account before considering rejecting the graft in borderline cases. (1B)
- The use of in situ normothermic regional perfusion and hypothermic machine perfusion can reduce the incidence of symptomatic ischaemic cholangiopathy when compared to static cold storage. (1A)
- Future studies comparing the effects of different machine perfusion technologies on the biliary tree in DCD donors as well as studies to find adequate assessment parameters of the viability of the biliary tree are necessary. (1A)
- The use of in situ normothermic regional perfusion is an effective way to increase the number of viable livers recovered. (1B)
- Future studies evaluating the possible mechanisms protecting against ischaemic cholangiopathy in DCD donors are needed. (1B)
- Excellent short- and medium-term outcomes can be achieved in paediatric DCD liver transplantation with highly selected and careful donor and recipient selection. (1B)
- Use of paediatric or young adult DCD grafts is an effective approach to expand the donor pool and remains an underutilised resource for children in need of liver transplantation. (1B)
- An international registry of paediatric DCD liver transplant recipients is needed to determine whether there is a significant difference in outcomes from DBD liver transplantation. (1B)
- A national differential analysis of outcomes in paediatric DCD liver transplantation is required, depending on whether a paediatric or an adult DCD is used. (1B)
We suggest that:
- DCD donors may be used without an age limit if other surrogates of donor organ quality are favourable. (2B)
- High donor BMI is a risk factor for graft loss both in DCD and DBD donation, therefore a higher BMI alone should not be a contraindication for accepting a DCD graft if other factors are favourable. (2B)
- Serial blood gases during the withdrawal phase should be used as an additional tool to determine the onset of anaerobic respiration by providing lactate measurements. (2D)
- The total preservation time could be extended beyond 8 hours if any of the machine organ preservation techniques are utilised, but there is no recommendation of a safe upper limit of preservation based on current evidence and this should be at the discretion of the implanting surgeon. (2D)
- Potential recipients of DCD liver grafts which have not been subject to in situ or ex situ perfusion should be informed of the potential risk of both early and late graft loss. (2C)
- The emphasis should be on minimising donor hepatectomy time; in ideal scenarios, hepatectomy time should not be longer than 30 min. A longer hepatectomy time is associated with graft failure in non-NRP DCD donors. (2C)
- Dual aortic and portal perfusion during DCD liver retrieval and flushing of the bile duct should be standard. (2C)
- Time between knife-to-skin and liver placement into the ice box should ideally be less than one hour; this is a target that all NORS teams should be encouraged to achieve. (2B)
- DCD livers can be used in children as whole, reduced or split grafts, if they are of excellent quality, the FWIT is <30 minutes and the CIT <8 hours. (2B)
- Paediatric DCD livers recovered without normothermic regional perfusion are less likely to present with ischaemic cholangiopathy as they may be more resilient to ischaemia reperfusion injury and have higher regenerative capacity. (2B)
- Machine perfusion in paediatric liver transplantation can play a role in halting the effects of the CIT, improving the liver quality in whole grafts for older children or young adults, and facilitating splitting and utilisation of both lobes. (2C)
Chapter 11 Pancreas
We recommend that:
- Pancreas transplantation from DCD donors offers similar outcomes to DBD donors, in terms of graft and patient survival, and therefore DCD donors should be considered an acceptable source of pancreatic grafts. (1B)
- Outcomes of DCD pancreas transplants are better with lower cold ischaemic times and, ideally, this should be kept to within 10 hours. (1B)
- Although DCD organs can be used for solitary pancreas transplantation, numbers are limited, and therefore most evidence supports their use for the simultaneous pancreas and kidney transplantation. (1C)
- Although arterial and venous thrombosis rates are similar between DCD and DBD pancreas transplants, appropriate systemic anticoagulation protocols should be considered. (1C)
We suggest that:
- Pancreas transplants from DCD donors are at increased risk of reperfusion pancreatitis and thrombosis and this may be exacerbated by prolonged cold ischaemia time >12 hours and increasing donor age >55 years. Ideal donors should be <45 years old and have a BMI <28 kg/m2. (2C)
- The pancreas team should stand down after a functional warm ischaemia time (systolic BP <50 mmHg) of 60 minutes, unless the pancreas is recovered using NRP, which may allow prolonged warm ischaemia time. (2C)
- There is limited evidence regarding the effect of recipient risk factors in terms of outcome after DCD pancreas transplantation, however, we would recommend considering the same risk factors as those that may contribute to an adverse outcome after DBD pancreas transplants (e.g., higher recipient BMI, cardiovascular morbidity, and technical surgical factors). (2C)
- Reported outcomes for DCD donor pancreas transplantation are broadly similar to those from DBD donors, although greater donor selection is likely to have taken place. (2C)
Chapter 12 Islets
We suggest that:
- Selection criteria for recipients of islets from DCD donors should be the same as for DBD donors. (2B)
- A pancreas recovered from a DCD donor should be allocated for islet isolation through the National Pancreas Offering Scheme. (2B)
- Satisfactory functional islet preparations can be routinely obtained from DCD donors and are as functional in vitro and after clinical transplantation as DBD islets. (2B)
- There is no difference in the long-term outcome of islet transplants from DCD donors when compared to DBD donors although the comparative cohort is small. (2C)
Chapter 13 Lung
We recommend that:
- DCD lungs should not be regarded as extended or marginal. Transplant survival and outcomes are at least similar to DBD organs. (1B)
- Pre-transplant ex vivo lung perfusion (EVLP) is advised in case of uncertain graft performance to safely extend donor and procedural criteria (e.g., long warm ischaemia, poor flush, clots). (1B)
- All patients on the lung transplant waiting list have the potential to receive DCD lungs. (1C)
We suggest that:
- The donor selection criteria for DCD lung transplantation should be the same as for DBD. (2B)
- Antegrade and retrograde flush perfusion should be performed at the time of lung retrieval. (2B)
- Organ acceptance criteria on EVLP may include measures of pulmonary compliance, vascular resistance, and gas exchange. (2C)
- If available, the use of EVLP as part of the organ retrieval and assessment processes should be considered. (2D)
Chapter 14 Heart
We recommend that:
- All patients on the heart transplant waiting list have the potential to receive DCD hearts. (1C)
We suggest that:
- The donor selection criteria for DCD hearts should be similar to those for DBD, except the age of the donor is currently 50 years or less. (2B)
3. Categorisation of DCD Donors and Definition of Warm Ischaemic Time
We recommend that:
- DCD donors should be categorised according to the Maastricht classification to aid research, communication and audit. (1B)
- The functional warm ischaemia time is defined as starting when the systolic blood pressure has a sustained (i.e., at least two minutes) fall below 50 mmHg, and extends up to the onset of cold in situ perfusion or normothermic regional perfusion. (1B)
3.1 Categorisation of DCD donors
Organ donation following circulatory death is classified as either ‘controlled’ or ‘uncontrolled’. Controlled donation occurs when life-sustaining treatment is withdrawn, often in an intensive care unit or anaesthetic room, and death follows. Uncontrolled donation refers to potential donors who suffer an unexpected cardiac arrest and are either brought into the hospital dead or when death is declared in the hospital following unsuccessful attempts at cardiopulmonary resuscitation (CPR).
DCD donors can be divided into categories based principally on work from the consensus meeting held in Maastricht in 1995 (1) and modifications made as a result of a meeting in Paris in 2013 (2). Classification is important both for the logistics of retrieval and analysis of the outcome following transplantation. Donation after circulatory death has become the term of preference; donation after cardiac death and non-heart-beating donation (NHBD) are terms that are no longer used, especially in the era of successful DCD heart transplantation.
The following classification is recommended:
Uncontrolled DCD donors
Maastricht Category 1: Found dead
1A. Out of hospital
1B. In hospital
This category includes sudden unexpected cardiac arrest without any attempt of resuscitation by a medical team. The circumstance would be that the patient is dead on arrival at the hospital and the setting is typically an emergency department.
Maastricht Category 2: Witnessed cardiac arrest
2A. Out of hospital
2B. In hospital
This category includes sudden unexpected irreversible cardiac arrest with unsuccessful resuscitation by a medical team. The setting is typically an emergency department.
Controlled DCD donors
Maastricht Category 3: Withdrawal of life-sustaining treatment with expected cardiac arrest
This category mainly refers to the decision to withdraw life-sustaining treatments. The planned WLST typically takes place in an intensive care unit or in the theatre suite.
Maastricht Category 4: Cardiac arrest while brain dead
Sudden cardiac arrest after brainstem death diagnosis but prior to planned organ recovery.
Legislation in some countries allows euthanasia (medically assisted cardiac arrest) and subsequent organ donation resulting in a fifth category. Euthanasia is illegal in the UK and Category 5 donors are not considered further in this document.
3.2 Nomenclature of Time Periods
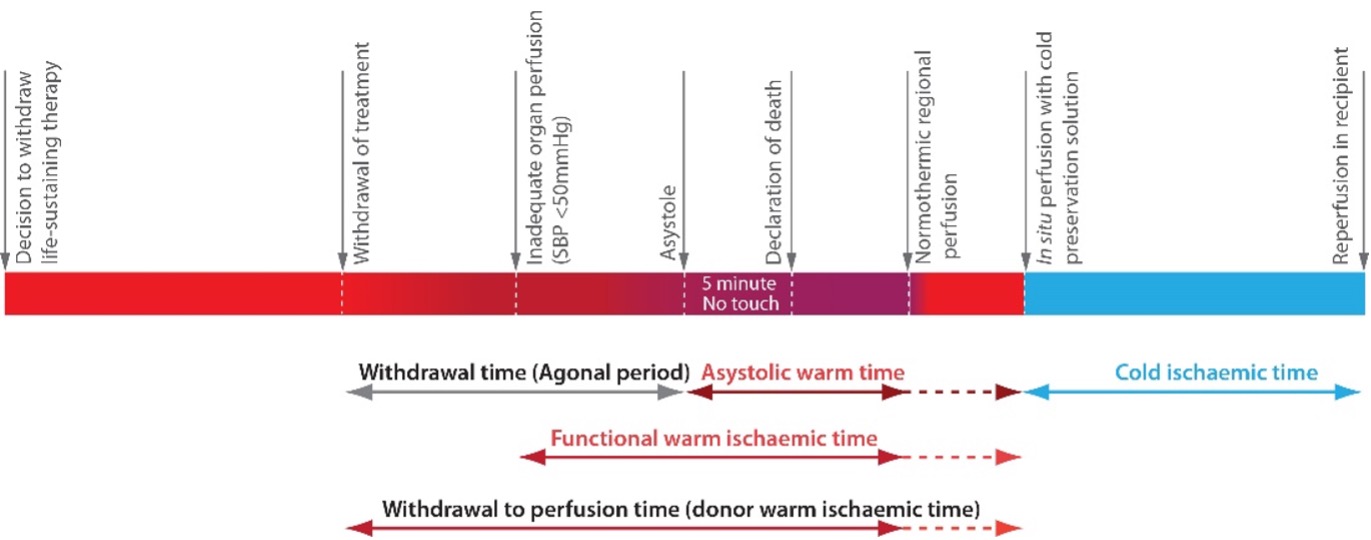
There is no standardised terminology for the nomenclature of time periods in DCD donation. In controlled Maastricht Category 3 DCD donors the process of organ donation starts with treatment withdrawal, following which the patient’s vital signs, in particular the blood pressure, deteriorate at varying rates until cardiac activity ceases (asystole). Following the verification of death, organ preservation begins with the perfusion of the donor with cold preservation solution or the commencement of normothermic regional perfusion (NRP). The following time periods are suggested and summarised in Figure 3.1.
- The withdrawal to perfusion time (sometimes called donor warm ischaemia time, DWIT) is defined as the time from donor treatment withdrawal to initiation of cold perfusion or the start of NRP.
- The functional warm ischaemia time (FWIT) is defined as the time when the systolic blood pressure has a sustained (i.e., at least two minutes) fall below 50 mmHg and extends up to the onset of cold in situ perfusion or commencement of NRP.
Figure 3.1. Suggested standardised terminology for the nomenclature of time periods in DCD donation.
Organs are particularly sensitive to warm ischaemia since metabolic processes continue but cells switch from aerobic to anaerobic metabolism. Anaerobic metabolism is heavily dependent on adenosine triphosphate (ATP) and intracellular ATP stores deplete rapidly. ATP is essential for the maintenance of membrane-associated ion exchange channels and, as warm ischaemia progresses, membrane integrity is lost, and cellular dysfunction and cell death occur. The same processes occur during cold ischaemia, but metabolism is slowed markedly under cold conditions, so cells and organs can survive for longer periods.
The FWIT reflects the detrimental effects of end-organ hypo-perfusion. Even though circulation exists, there is a blood pressure at which perfusion is suboptimal and warm ischaemia is considered to have begun. This threshold pressure will vary from donor to donor, depending on factors such as age, and is defined differently in different countries; for the purpose of these guidelines it is defined as beginning when the systolic blood pressure is below 50mmHg. It is therefore appropriate to also consider this period of warm ischaemia when assessing likely organ damage, rather than only the asystolic warm period (i.e., the time from circulatory arrest to the perfusion of the organs with cold preservation solution or commencement of NRP).
Although there is little published evidence to support using a systolic pressure of 50mmHg as the start point for FWIT, the definition has been incorporated into the UK DCD Risk Score for liver donors that has been validated using both the UK and the Scientific Registry of Transplant Recipients registry cohorts (3).
It should also be appreciated that organs from younger donors are likely to tolerate hypotension far better than older donors, and organs from patients who have a history of hypertension are likely to experience significant critical ischaemia even at systolic blood pressures in excess of 50 mmHg (4).
The time for which the haemoglobin oxygen saturation is below 70% or 80% can also be considered (5). Low donor oxygen saturation may be used by some when assessing the suitability of some donor organs but without solid evidence base. It has long been accepted that the accuracy of peripheral pulse oximetry is limited in the setting of severe hypoxia or hypotension (6,7). As a result, the current recommendation is that oxygen saturation should not be used as an indicator of poor outcomes or as a reason for non-utilisation of all organs. It has been suggested that further research on organ-specific outcomes and low donor oxygen saturation is needed (7). An alternative way to determine suboptimal organ perfusion is to perform a blood gas and see whether the lactate has risen, a sign of anaerobic metabolism.
In the era of NRP and ex situ machine perfusion, the potential for organ viability testing may reduce the need for further research into the utility of donor oxygen saturations.
References
- Kootstra G, Daemen JH, Osmen AP. Categories of non-heart-beating donors. Transpl Proc 1995;27:2893-4.
- Thuong M, Ruiz A, Evrard P, et al. New classification of donation after circulatory death donors definitions and terminology. Transpl Int 2016;29(7):749-59.
- Schlegel A, Kalisvaart M, Scalera I, et al. The UK DCD Risk Score: A new proposal to define futility in donation-after-circulatory-death liver transplantation. J Hepatol 2018;68(3):456-64.
- Bernat J, Capron A, Bleck T, et al. The circulatory-respiratory determination of death in organ donation. Critical Care Medicine 2010;38:972-9.
- Kalisvaart M, Croome KP, Hernandez-Alejandro R, et al. Donor warm ischemia time in DCD liver transplantation-working group report from the ILTS DCD, liver preservation, and machine perfusion consensus conference. Transplantation 2021;105(6):1156-64.
- Rutherford KA. Principles and application of oximetry. Critical Care Nursing Clinics of North America 1989;1(4):649-57.
- Croome KP, Barbas AS, Whitson B, et al. American Society of Transplant Surgeons recommendations on best practices in donation after circulatory death organ procurement. Am J Transplant 2023;23(2):171-9.
4. Diagnosis of Death
We recommend that:
- The decision to withdraw life-sustaining treatment and criteria for the diagnosis of death must not be influenced by the possibility of subsequent organ recovery. (1A)
- The process of controlled DCD must be consistent with the provision of high-quality end-of-life care for a dying patient and their family. (1A)
- Death is defined as the permanent loss of the capacity for consciousness and loss of all brainstem functions. This may result from the permanent cessation of circulation and/or after catastrophic brain injury. Death is confirmed using circulatory criteria in the former and neurological criteria in the latter. (1A)
- In the context of death determination, ‘permanent’ refers to loss of function that cannot resume spontaneously and will not be restored through intervention. (1A)
- Where circulatory criteria are used, death can be confirmed after five minutes of continuous cardio-respiratory arrest, providing there is no subsequent intervention with the potential to restore cerebral perfusion. (1A)
- Where possible, circulatory arrest should be identified and monitored by the absence of pulsatile flow on a correctly functioning arterial line or using echocardiography if the expertise is available; or failing that, by continuous ECG monitoring. (1C)
- DCD organ recovery protocols should recognise the potential risks around post-mortem interventions that might restore cerebral perfusion and implement strategies to prevent this. (1C)
4.1 Professional Frameworks for the Diagnosis and Confirmation of Death
Confirming death has clinical, legal and societal implications. Consequently, although there is increasing international professional consensus on the biological features that can and should be considered to be a state of death, other factors determine how these separate elements are assimilated into the professional and/or legal frameworks that clinicians are required to apply within a given jurisdiction. Invariably, differences emerge as country-specific criteria are developed and these become most evident when incorporated into deceased donor organ recovery protocols. This is evidenced by the variation in waiting or observation periods from the onset of loss of vital functions to the confirmation of death across different countries (1,2).
The definition of death applicable in the United Kingdom, and accepted in Case Law, is authoritatively provided by the Academy of Medical Royal Colleges 2008 Code of Practice for the Diagnosis and Confirmation of Death (3). This defined death as:
“The irreversible loss of the capacity for consciousness, combined with irreversible loss of the capacity to breathe. This may be secondary to a wide range of underlying problems in the body, for example, cardiac arrest.”
The Academy’s guidance went further to state that after diagnosing death using circulatory criteria:
“It is obviously inappropriate to initiate any intervention that has the potential to restore cerebral perfusion after death has been confirmed.”
This in effect recognised that death can, and usually is, confirmed at the point of permanent loss of the circulation to the brain as defined above.
Internationally, a World Health Organization collaboration has attempted to develop a consensus on the scientific, biological and medical aspects of death in a way that is hoped to supersede international differences, and which may form the basis of more consistent and globally applicable diagnostic criteria (4). The consensus group defined death as occurring
“When there is permanent loss of the capacity for consciousness and loss of all brainstem functions. This may result from permanent cessation of circulation and/or after catastrophic brain injury. In the context of death determination, ‘permanent’ refers to loss of function that cannot resume spontaneously and will not be restored through intervention.” (4)
This international definition is entirely consistent with the UK definition but offers some advantages because of its clarity in terminology. These definitions mean that:
- Death is a biological process and should be diagnosed on such a basis.
- The criteria used to diagnose death are based on loss of specified brain functions rather than anatomically based (cardiac death, whole brain death or brainstem death)
- There is only one brain-based definition of death, and this can be confirmed using circulatory criteria following a cardiorespiratory arrest or using neurological criteria following a catastrophic brain injury.
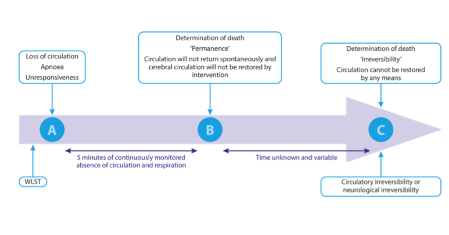
- Death can be diagnosed using circulatory criteria at the point of permanence. This is the point beyond which the circulation will not return spontaneously and will not be restarted through intervention because a decision has been made not to attempt to do so.
- This point of permanence is reached in less than five minutes of continuous cardiorespiratory arrest following the WLST in all individuals (5).
- Diagnosing death at the point of permanence is equivalent to the point of irreversibility (brain function cannotbe restored through intervention) only if no intervention with the potential to restore brain perfusion is undertaken after the confirmation of death.
- Any post-mortem intervention that restores cerebral perfusion potentially invalidates the diagnosis of death and jeopardises the integrity of the ‘dead donor’ rule.
- Organ recovery teams must understand their responsibilities in this regard.
- DCD protocols must ensure that the criteria used to diagnose death remain valid during the duration of the organ recovery procedure.
- National protocols that mitigate against the risks of restoring cerebral perfusion are available from NHS Blood and Transplant and must be followed in all cases of DCD organ recovery.
- Specific UK guidance is available for DCD lung retrieval (6) and for procedures using in situ normothermic regional perfusion, either abdominal or thoraco-abdominal (7,8).
4.2 Biological Background to Death that Follows Permanent Loss of the Circulation after WLST
Neurological and circulatory functions are inextricably linked. Death, defined as the loss of capacity for consciousness and all brain stem function, most commonly occurs following the loss of circulatory function that will not be, or cannot be restored. The terms ‘permanent’ (will not be restored) and ‘irreversible’ (cannot be restored) loss of function have been used to distinguish between these two groups (4,9). It is important to understand that without brain blood flow there can be no brain perfusion and without brain perfusion, there can be no brain function. Since the diagnosis of death is based on the permanent loss of brain functions, then it is the permanent loss of the circulation to the brain that is crucial to the diagnosis.
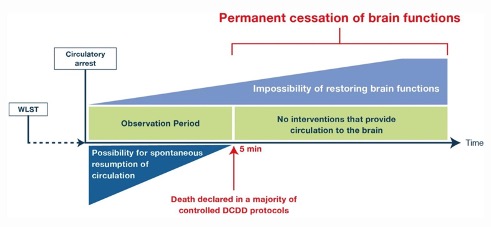
Following cardio-respiratory arrest loss of consciousness occurs within 21 seconds, the electroencephalogram becomes isoelectric within 30 seconds and all evoked potentials are lost in less than 5 minutes (10). Brain function cannot resume unless circulation to the brain returns spontaneously (autoresuscitation) or through intervention. In a large prospective observational study, the longest recorded time to spontaneous return of the circulation following cardiorespiratory arrest after the WLST was 4 minutes and 20 seconds (5). Therefore, death can be diagnosed based on the permanent loss of brain function after five minutes of continuously monitored cardio-respiratory arrest. This diagnosis is conditional on the prohibition of any post-mortem interventions that may restore brain blood flow (Figure 4.1).
Figure 4.1. Death is determined by the permanent cessation of brain function following circulatory arrest. The x-axis in time is not linear. The time that elapses from 5 min when death is declared by the permanent absence of circulation to assured irreversibility of brain functions is in hours. DCDD= donation after the circulatory determination of death, WLST = withdrawal of life-sustaining treatment. (Reproduced with permission from reference 11; see https://link.springer.com/article/10.1007%2Fs00134-020-06341-7#rightslink)
The time that must elapse after cardio-respiratory arrest to ensure that the brain will not respond to subsequent restoration of brain circulation is unknown. It is likely to be influenced by multiple variables that are difficult to control e.g., temperature, patient co-morbidities, the duration of hypoperfusion and subsequent absent brain circulation before its restoration and the effectiveness of cardiopulmonary resuscitation. It is possible that irreversible loss of brain function may occur within the first five minutes after circulatory arrest (Figure 4.1). However, the time required from the confirmation of death at the point of permanent loss of circulatory function to assured irreversibility of loss of brain functions is potentially measured in hours (11).
4.3 Practicalities in Diagnosing Death after WLST
There are three mandatory, consecutive steps involved in diagnosing death in the context of controlled DCD:
- A decision is reached to WLST and not to initiate cardiopulmonary resuscitation after asystole. Both should be documented in the patient’s medical notes.
- A full five minutes of observation and monitoring to confirm the continuous absence of the circulation, apnoea and unconsciousness; after which the time for possible autoresuscitation will have elapsed.
- An absolute prohibition of post-mortem interventions that could restore brain perfusion.
It is a fundamental principle that members of the transplant team are not involved in any decision to WLST in a potential organ donor nor in decisions regarding actions involved in the WLST (11). The decision is reached by the multidisciplinary ICU team, in consultation with the patient or their family. The decision is always made in the best interest of that patient and in accordance with national professional guidance (12). Members of the transplant team must not be involved in the diagnosis and confirmation of death in eligible donors undergoing the WLST.
There is currently no consensus on how the treatment should be withdrawn irrespective of whether the patient consented to DCD or not. It is however common practice for patients to be extubated and for sedative medications to be used when required and titrated to ensure patient comfort. The practice of controlled DCD must be consistent with the provision of high-quality end-of-life care for a dying patient.
Following the WLST and the subsequent loss of the circulation and apnoea, five minutes of observed and monitored continued absence of the circulation and apnoea are required to make the diagnosis of death. Any return of the circulation or respiratory effort should mean that the five minutes observation period is restarted at the point when the circulation is lost again (Figure 4.2). After five minutes have elapsed, the potential for a spontaneous return of the circulation has passed.
Absence of the circulation can be confirmed by the demonstration of mechanical asystole using an arterial line and continuous intra-arterial pressure monitoring. An alternative approach is the use of transthoracic echocardiography if the expertise and equipment are available (Figure 4.2). While electrical asystole on ECG will confirm concomitant mechanical asystole, waiting for electrical asystole will prolong warm ischaemia and is therefore not recommended if another modality is available.
At the end of the five minutes, a brief neurological examination is performed to confirm the loss of the corneal and pupillary light reflexes and no motor response to supraorbital pressure (Figure 4.2).
After the five minutes have elapsed it is still possible to restart the heart, and the circulation can be restored using extracorporeal technique even if the heart is not restarted. Indeed, both form the basis of normothermic regional perfusion of the thoracic and abdominal organs. It is also known that after five minutes of asystole, it may still be possible to restore brain function if cardio-pulmonary resuscitation or extracorporeal assisted cardiopulmonary resuscitation is started. The principle of permanence of death diagnosis must be maintained by ensuring that brain perfusion is not restored and using techniques to prevent this from happening (8). Any restoration of brain perfusion may interrupt the dying process and invalidate the diagnosis of death.
References
- Controversies in the Determination of Death: A White Paper by the President’s Council on Bioethics. US Government Printing Office 2011. ISBN 0160879035, 9780160879036.
- Dhanani S, Hornby L, Ward R, et al. Variability in the determination of death after cardiac arrest: A review of guidelines and statements. J Intensive Care Med 2012;27:238-52.
- Academy of the Medical Royal Colleges. A Code of Practice for the Diagnosis and Confirmation of Death. London 2008. Available at: http://aomrc.org.uk/wp-content/uploads/2016/04/Code_Practice_Confirmation_Diagnosis_Death_1008-4.pdf .
- Shemie SD, Hornby L, Baker A, et al, and The International Guidelines for Determination of Death phase 1 participants, in collaboration with the World Health Organization. International guideline development for the determination of death. Intensive Care Med 2014;40:788-97.
- Dhanani S, Hornby L, van Beinum A, et al. Resumption of cardiac activity after withdrawal of life-sustaining measures. N Engl J Med 2021;384:345-52.
- NHS Blood and Transplant 2018. Care of Potential Lung DCD Donors – Safety Brief. Available at: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/12279/care-of-potential-lung-dcd-donors-inf1425.pdf .
- NHS Blood and Transplant 2021. UK Protocol for Normothermic Regional Perfusion (NRP) in controlled Donation after Circulatory determination of Death. NRP National Protocol. Available at: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/23872/uk-protocol-for-normothermic-regional-perfusion-version-142-29-06-21.pdf .
- Manara A, Shemie SD, Large S, et al. Maintaining the permanence principle for death during in situnormothermic regional perfusion for donation after circulatory death organ recovery: A United Kingdom and Canadian proposal. American Journal of Transplantation 2020:20:2017-2025.doi: 10.1111/ajt.15775. Epub 2020 Jan 27
- Bernat J, Capron A, Bleck T, et al. The circulatory-respiratory determination of death in organ donation. Critical Care Medicine 2010;38:972-9.
- Pana R, Hornby L, Shemie SD, et al. Time to loss of brain function and activity during circulatory arrest. J Crit Care 2016; 34:77–83. https://dx.doi.org/10.1016/j.jcrc.2016.04.001
- Domínguez-Gil B, Ascher N, Capron AM, et al. Expanding controlled donation after the circulatory determination of death: statement from an international collaborative. Intensive Care Med 2021;47:265-81 https://doi.org/10.1007/s00134-020-06341-7
- General Medical Council 2010. Treatment and care towards the end of life: good practice in decision making. Available at: https://www.gmc-uk.org/ .
5. Law, Ethics and Donor Consent
We recommend that:
- ‘Overall benefit’ should be the guiding principle when making decisions about end-of-life care in relation to organ donation. (1D)
- The strength of a patient’s wishes and decisions regarding organ donation should be included in any consideration of overall benefit. (1D)
- It should not be assumed that someone opting-in to organ donation necessarily has a stronger wish to donate their organs than someone who did not record a preference. (1D)
- Appropriate frameworks/guidance (‘Donation Actions Framework’ in England, Wales and Northern Ireland; ‘Guidance on the authorisation and undertaking of pre-death procedures’ in Scotland) should be used to guide decision-making regarding what actions are ethically and legally permissible in the context of DCD. (1D)
- Further consideration is given to ethical considerations related to undertaking organ donation research, particularly in relation to consent for such activities. (1D)
5.1 Key Ethical Considerations in DCD – The Importance of Patients’ Wishes
5.1.1 Overall Benefit
The ethically distinctive aspect of DCD donation is that the patient is alive, albeit in most cases lacking capacity, when decisions about, and preparations for, organ donation are made. The usual ethical and legal framework for making treatment decisions in the absence of capacity, therefore, applies, which means decisions regarding treatment and end-of-life care should be made on the basis of overall benefit to the patient in line with GMC guidance on end-of-life care (1). We will use the term ‘overall benefit’ rather than ‘best interests’ to refer to the ethical basis and guiding principle on which decisions are made about treatment/care for adult patients who lack capacity to make decisions for themselves. This ensures consistency with relevant legislation across all devolved nations. This introduces potential complexity when organ donation is considered, as organ donation is often characterised as something done to benefit people other than the donor.
There are different perspectives to consider when determining overall benefits. There is a medicalised perspective that weighs the medical harms and benefits that different treatment options may offer. This medicalised perspective weighs heavily in decisions about WLST. The medicalised perspective is, however, incomplete. Determining what is, on balance, best for somebody also requires consideration of a person’s wishes, values and beliefs – things that are very clearly beyond the medical perspective.
Something might be beneficial to a person if it helps them to achieve their goals, or contrariwise harmful if it prevents them from achieving their goals. Accordingly, what counts as a benefit (or harm) can vary from person to person. The ethical principle of respect for autonomy makes the wishes of a patient a central consideration in determining what is best for that patient, and this principle is reflected implicitly in mental capacity legislation. Respecting the autonomous wishes of a patient with respect to legitimate goals can be considered to provide them with benefits and frustrating these, harm. The benefits and harms of respecting a patient’s wishes need to be weighed against other benefits and harms (such as ‘medical’ benefits and harms), so it is not the case that always and simply respecting a patient’s wishes necessarily provides overall benefit. Where respecting a patient’s wishes will provide some significant benefit to a patient, and the harms of respecting those wishes are low, however, respecting those wishes can be considered to provide overallbenefit.
The benefits to a deceased donor of donating their organs may be largely abstract or symbolic, as they cannot experience them, but they are nonetheless considered important. Being remembered positively for undertaking a final generous, altruistic act or giving one’s loved ones something positive to take from a difficult situation are examples of the types of benefits that may accrue to donors beyond just the benefit of having their wishes respected. Additionally, knowing during life that one may be able to help others after one’s death may be considered a benefit.
Given these benefits to donation, if a dying patient wishes to become an organ donor, then taking action to facilitate organ donation may be considered to provide some overall benefit. Any interventions, though, must be balanced against the harms they may cause, as well as the potential harm of frustrating the patient’s wishes to be a donor.
5.1.2 Guiding Principles
The UK Donation Ethics Committee (UKDEC) endorsed two guiding principles in their work (2), and these are used in this chapter to underpin the ethical aspects of DCD:
Principle 1: Where donation is likely to be a possibility, full consideration should be given to the matter when caring for a dying patient
Principle 2: If it has been established that further life-sustaining treatment is not of overall benefit to the patient, and it has been further established that donation would be consistent with the patient’s wishes, values and beliefs, consideration of donation should become an integral part of that patient’s care in their last days and hours.
These principles highlight the importance of establishing a patient’s wishes, in relation to their end-of-life care in general, and specifically how organ donation might form a part of this.
5.2 Legal Aspects of DCD – Establishing and Acting Upon Patients’ Wishes
5.2.1 Capacity Legislation
Relevant legislation for making decisions for adult patients who lack capacity differs according to country. Mental Capacity Act 2005 applies in England and Wales (3), the Adults with Incapacity (Scotland) Act 2000 applies in Scotland (4), and the Mental Capacity Act (Northern Ireland) 2016 applies in Northern Ireland (5). Although there are differences in precise terminology, the guiding principle of each is the promotion of ‘overall benefit’. Each piece of legislation also makes clear that decisions should take into account the wishes and feelings of the patient when determining what constitutes overall benefit.
The possibility of DCD arises only once it has been determined that maintaining life-sustaining treatment is not providing overall benefit to a patient and that such treatment should therefore be withdrawn. As this decision would be made regardless of the possibility of organ donation, it will not be the focus of discussion here. Of greater relevance here is the relationship between a person’s wishes regarding organ donation, their end-of-life care and overall benefit.
5.2.2 Organ Donation Legislation
England (6), Scotland (7), Wales (8) and Northern Ireland (9) have all adopted systems of deemed consent for organ donation. Consent for organ donation is defined within the legislation applicable to each devolved nation, and although each has minor differences, they all provide a way of establishing a patient’s wishes in relation to organ donation. In summary, under these deemed consent systems people can do different things to record their organ donation wishes. First, even though these are known as opt-out systems, it is still possible to opt-in and record a positive wish and ‘express consent’ to become an organ donor (and to state specific organs one would be willing to donate). Second, if one does not wish to become an organ donor, one can opt-out. Third, one can do nothing: in this final scenario it is presumed that a lack of action implies consent/authorisation for donation, provided certain conditions are met.
As stated earlier, when it has been determined that life-sustaining treatment is not offering overall benefit, and it has been determined that organ donation is consistent with the patient’s wishes, consideration of organ donation should become an integral part of the patient’s care. The difficult ethical question here is how the pursuit of organ donation should be weighed against other important considerations at the end-of-life.
Views about becoming an organ donor can vary. Some people may feel very strongly about donating and helping others may be at the core of their identity. Others may not hold strong views about donating, but nonetheless see few downsides and therefore be marginally overall in favour. When someone has a strong wish to become a donor (or to look at it another way, when becoming an organ donor is particularly important to someone) becoming a donor can be considered to offer them significant benefit. If someone was known to be largely indifferent to donation but nonetheless had not opted out, it is likely that they had a much weaker wish to become a donor, then donation is less important to them and consequently is likely to offer less significant benefit.
5.2.3 ‘Strength of Evidence’ Versus ‘Evidence of Strength’
It may be tempting to assume that someone opting-in to organ donation has a stronger wish to donate than someone who has not opted out, since they have taken positive action to record their wish. It is important to draw a distinction, however, between ‘strength of evidence’ of a wish to donate, and ‘evidence of strength’ of a wish to donate (10). A positive action to express consent and opt-in may provide the clearest evidence of a wish to become a donor, but it does not provide definitive proof of a strong wish to donate. Similarly, in a situation where express consent may be absent and consent is deemed, this does not necessarily imply a weaker wish to donate. ‘Evidence of strength’ of a wish to donate may be best determined through sensitive conversation with those who know the donor best to gain a fuller understanding of a patient’s values and beliefs with respect to donation.
The strength of the wish to donate, or the importance of donation to a particular patient, is a vital consideration when determining what is of overall benefit to that patient. Because the benefits and harms of particular courses of action to facilitate donation have to be weighed against the benefits and harms of donation proceeding or not, more intrusive actions to facilitate donation may be more permissible for patients with strong wishes to become donors than those with weak wishes.
5.3 Resolving Conflicting Duties
The prospect of DCD organ donation can give rise to multiple potential conflicts, which require careful consideration. The first significant potential conflict arises with the decision to WLST. This initial decision must never be made in conjunction with decisions about organ donation, or decisions about organ donation made prior to this decision. It must remain clear to patient’s families and the wider public that the potential for organ donation never leads to treatment being withdrawn.
5.3.1 Potential for Harm
A further potential conflict arises if actions that might make it possible or increase the likelihood of organ donation proceeding, run the risk of causing harm to the patient. For example, organ donation may require delaying the WLST, even though it has been determined previously that life-sustaining treatment is not providing an overall benefit to the patient. Healthcare professionals have a duty to not harm their patients and continuing with interventions that are not resulting in benefit – given that there may be some burden associated with them – could be construed as harmful. Patients can be harmed in different ways, however, and these potentially different harms need to be balanced.
Some procedures run the risk of harming a patient if, for example, they are likely to cause pain and suffering or actively hasten a patient’s death. Patients may also be considered harmed if their wishes are unnecessarily frustrated. Herein arises the key tension: continuing with interventions that are no longer providing benefits, may run the risk of causing harm to a patient but may be necessary to avoid frustrating the patient’s wish to become an organ donor. It is unlikely that any intervention posing a risk of distress or serious harm to the patient would be considered to provide overall benefit regardless of the impact on organ donation, but other interventions may fall into a category of permissibility. Each action to facilitate donation should be considered in terms of its harms and benefits, and in light of the strength of the patient’s wish to donate, to determine whether it is likely to contribute to overall benefit. This extends to interventions necessary to maximise the prospects of successful donation and/or transplantation.
5.3.2 Maximising Prospects of Successful Transplantation
It is reasonable to assume that individuals who wish to donate organs, or who are presumed not to object to organ donation, wish for donation to bring benefit to others. Accordingly, the wish to be a donor is not wholly fulfilled by the removal of organs, nor transplantation of those organs into another’s body: the wish is that those donated and transplanted organs will extend, or improve the quality of the life of the recipient. Equally, recipients agree to be transplanted not to fulfil the donor’s wishes but because transplantation offers them the best prospects for a longer and/or better quality of life. In this respect the wishes of recipients and donors are aligned; both wish for a successful transplantation where success is measured in terms of the survival and effective functioning of the transplanted organ.
Successful transplantation can never be guaranteed, but measures that will make this more likely include interventions undertaken before death is declared and whilst the organs are still in situ in the donor. Such measures could be perceived as generating another conflict of interest since they are interventions done to one patient to benefit another. The resolution here also turns on the strength of the donor’s wishes to donate. There is a risk that if measures to improve the prospects of successful transplantation are not taken, donation will not proceed because the condition of the organs will be too poor. On the one hand, it is wrong to assume that a willingness to be a donor encompasses anything and everything necessary for the donation to be successful. Decisions made for a patient’s overall benefit, taking into account information provided by those who knew the donor best, have to be made on an intervention-by-intervention basis. A potential DCD donor remains under the protection of mental capacity legislation, and this protection is not weakened by considerations of the overall benefit to potential recipients save insofar as these are harmonious with the interests of the donor.
Potential recipients and their surgeons must make their own decisions about whether to proceed based on the quality of organs offered for donation. The potential donor is not wronged if transplantation does not proceed provided reasonable efforts have been made to facilitate their wishes compatible with the overarching duty to act for their overall benefit. Similarly, they are not wronged if there are contraindications to donation (11).
Potential organ recipients will understandably hope to secure good quality organs. For many, the quality of the organ will be a secondary consideration to the primary concern of being offered any organ while they are still well enough to benefit from transplantation. Most deceased donor organs are allocated nationally (12) and therefore the ethical implications of allocation models are the domain of the national bodies charged with keeping them under review. Recently, deceased donor kidney allocation models have, where possible, attempted to align organ quality with recipient risks. Recipients do not have to accept an organ just because it has been offered, however. Some recipients who do not require immediate transplantation may choose to balance the risks of accepting a ‘higher risk’ organ against the risk of waiting for a better alternative, which may or may not materialise (see Chapter 8). Permitting recipients to make these choices may come at a cost to others on the transplant waiting list in part because delays in allocating a deceased organ can increase its risk rating, and also because when potentially transplantable organs are not allocated, the waiting list, and therefore waiting time, is not reduced. Moreover, further progress in overall transplantation depends on pushing the boundaries with riskier organs and learning how to reduce and mitigate the risks.
5.3.3 Duties to Relatives
There may also be conflict between duties to the patient and perceived duties to the patient’s relatives. Although there is much ethical literature arguing against the so-called ‘family veto’, it has historically been the case that organ donation will not generally proceed unless family members agree. The NHSBT website makes it clear that clinicians will never proceed with organ donation if a patient’s family objects (13), which potentially puts patient wishes in conflict with the wishes of their family. In some instances, this conflict may also be resolved by giving more holistic consideration to overall benefit: potentially willing donors may not wish to proceed with donation that it was going to cause distress to their relatives, in which case not going ahead with the donation better respects their wishes. This is unlikely to be universally true, however, so each case will require careful consideration of the benefits and burdens to the potential donor and their family, and the available evidence of the strength of wishes to donate.
5.3.4 Donation Actions Framework
A framework has been developed to support consideration of which ‘donation actions’ are likely to be permissible in England, Wales and Northern Ireland (14). This framework defines ‘donation actions’ as “activities or interventions carried out in relation to a potential organ donor, either before or after death, for the purpose of exploring donation eligibility, facilitating deceased organ donation, increasing organ utilisation, and/or optimising transplant outcomes”. We recommend that this guidance be used to provide a systematic and structured approach to supporting decisions about what may or may be permissible in the context of DCD. Scotland has its own guidance, and we recommend that this is used in Scotland (15).
5.4 Use of Organs for Research
Research related to organ donation can be split into two broad categories: i) research involving donated organs and ii) research involving the organ donor. The latter arguably raises more complex ethical issues than the former, particularly in cases of DCD where the donor may still be alive at the time research participation is being considered. Ethical aspects of organ donation research remain the subject of debate and are more complex than some other types of research. For example, there are multiple stakeholders to consider, including the donor, their relatives, and potential recipients. Recent literature has highlighted the need for specific guidance for research related to organ donation (16), as well as the need for further empirical work exploring appropriately context-sensitive consent models (17). NHS Blood and Transplant provide a detailed Research Process Handbook which covers the practical aspects of undertaking research, including detailed guidance on consent requirements (18).
5.5 BTS Ethics Committee
The Ethics Committee is a BTS sub-committee of multidisciplinary healthcare professionals practising in transplantation and its related fields. It consists of elected and appointed individuals with specialist expertise in ethical issues that are relevant to donation and transplantation. The committee encourages questions and approaches for advice or help of any kind in the area of transplantation ethics and can be contacted via ethics@bts.org.uk or through the officers of the BTS.
References
- General Medical Council. 2010. Treatment and care towards the end of life: good practice in decision making. Available at: https://www.gmc-uk.org/-/media/documents/treatment-and-care-towards-the-end-of-life—english-1015_pdf-48902105.pdf.
- UK Donation Ethics Committee 2011. An Ethical Framework for Controlled Donation After Circulatory Death. Available at: https://www.aomrc.org.uk/wp-content/uploads/2016/05/Controlled_donation_circulatory_death_consultation_0111.pdf.
- Mental Capacity Act 2005. Available at: https://www.legislation.gov.uk/ukpga/2005/9/contents.
- Adults with Incapacity (Scotland) Act 2000. Available at: https://www.legislation.gov.uk/asp/2000/4/contents.
- Mental Capacity Act (Northern Ireland) 2016. Available at: https://www.legislation.gov.uk/nia/2016/18/contents/enacted.
- Organ Donation (Deemed Consent) Act 2019. Available at: at: https://www.legislation.gov.uk/ukpga/2019/7/contents/enacted.
- Human Tissue (Authorisation) (Scotland) Act 2019. Available at: https://www.legislation.gov.uk/asp/2019/11/contents.
- Human Transplantation (Wales) Act 2013. Available at: https://www.legislation.gov.uk/anaw/2013/5/contents.
- https://www.nidirect.gov.uk/articles/organ-donation.
- Gathani A, Moorlock G, Draper H. Pre-mortem interventions for donation after circulatory death and overall benefit: A qualitative study. Clinical Ethics. 2016 Dec;11(4):149-58.
- NHS Blood and Transplant. 2021. POL188/14 – Clinical Contraindications to Approaching families for Possible Organ Donation. Available at: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/25143/pol188.pdf.
- NHS Blood and Transplant. 2021. POL200/5 – Introduction to Patient Selection and Organ Allocation Policies. Available at: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/25146/pol200.pdf.
- NHS Blood and Transplant. Understanding consent for organ donation. Available at: https://www.organdonation.nhs.uk/helping-you-to-decide/about-organ-donation/consent/.
- Intensive Care Society, NHS Blood and Transplant and partners. 2022. Donation Actions Framework. Available at:https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/27065/donation-actions-framework-v10-june-2022.pdf.
- Scottish Government, Chapter 8: Guidance on the authorisation and undertaking pre-death procedures. Available at: https://www.gov.scot/publications/guidance-deceased-organ-tissue-donation-scotland-authorisation-requirements-donation-pre-death-procedures-1st-edition-published-march-2021-1st-ed/pages/8/.
- Martin DE, Cronin AJ, Dalle Ave A, et al. Addressing ethical confusion in deceased donation and transplantation research: the need for dedicated guidance. Transplant International. 2021 Dec;34(12):2459-68.
- Cooper J, Harvey D, Gardiner D. Examining consent for interventional research in potential deceased organ donors: a narrative review. Anaesthesia. 2020 Sep;75(9):1229-35
- NHS Blood and Transplant. 2021. ODT Research Process Handbook. Available at: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/26740/inf1393.pdf.
6. Organ Retrieval
We recommend that:
- Treatment withdrawal should ideally be planned for a time when the donor HLA type and virology are known and the thoracic, liver and pancreas organ offers have been accepted or declined by recipient centres. (1C)
- The logistics to achieve a shorter asystolic warm period after confirmation of death should be discussed by the NORS team with the SNOD. Treatment withdrawal in the operating department is associated with a shorter asystolic warm withdrawal in a remote intensive care unit. (1C)
- The retrieval team need to be satisfied with the donor details (blood group, past medical history, illness leading to death, etc) before treatment is withdrawn. (1A)
- Retrieval teams should be prepared in the operating theatre at the point of treatment withdrawal. (1B)
- In Maastricht 4 donors, where death has been established previously by neurological criteria, heparin (or other appropriate treatments) may be given just prior to treatment withdrawal. (1A)
- In Maastricht 4 donors, where death has been established previously by neurological criteria, death does not need to be reaffirmed once circulatory arrest has occurred, but five minutes of continuous cardio-respiratory arrest should still be observed. (1A)
- For controlled donors, retrieval starts by gaining access to a large artery and vein, typically the right common iliac artery or aorta, and the IVC in the abdomen or right atrium in the chest. (1B)
- Cannulae for infusion of preservation fluid should not be placed in the SMV or IMV when the pancreas is being retrieved. (1B)
We suggest that:
- There is no substantive evidence for adding a fibrinolytic agent such as streptokinase or recombinant tissue plasminogen activator to the preservation solution. (2B)
- Heparin should be added to the first two litres of preservation solution to be perfused through the aorta and portal vein at a dose of 300 IU/kg donor bodyweight (around 25,000 IU for an 80kg person). (2B)
- The liver should be recovered using a rapid technique that minimises liver congestion. (2C)
- Dual perfusion of the hepatic artery and portal vein is preferred for recovery of DCD livers, apart from in infant donors of <15kg body weight. (2C)
- The pancreas may be removed either en bloc with the liver, or separately from the liver. (2D)
- En bloc removal is the preferred technique for retrieval of small paediatric kidneys (aged under 5 years old). In larger paediatric donors, the kidneys may be removed either individually or en bloc.(2D)
6.1 The Procedure of Organ Recovery
6.1.1 Timing of Treatment Withdrawal: General Considerations
There are several screening tests (e.g., donor virology) and other tests (e.g., human leucocyte antigen (HLA) typing) that should be completed urgently.
Allocation of kidneys, pancreas and lungs usually require the donor HLA type to be known, thereforedonor blood should be sent to the nearest histocompatibility and immunology laboratory as soon as possible. Additional blood may be requested by the recipient centres ahead of withdrawal to allow apre-emptive crossmatch to be performed on peripheral blood.
Once the intended recipients have been identified, it is desirable that the heart, lung, liver andpancreas recipients are all in the recipient hospitals when treatment withdrawal in the donor is planned.
Good communication between the retrieval team and local team should be upheld. The Specialist Nurse in Organ Donation (SNOD) should act as the key person to communicate and plan the process with the NORS team.
The NORS team/s are responsible for reviewing the potential donor’s hospital/medical notes to satisfythemselves that they have the correct information, preferentially using a standard surgical safety checklist. In particular, checks should take place with respect to donor identity, donor blood group, virology of the donor and past medical history.
6.1.2 Preparations for Treatment Withdrawal
To minimise warm ischaemia injury during the time between cardio-respiratory arrest and perfusion with ice-cold preservation solution (or initiation of NRP), donor transport from the location of WLST to the operating table should be kept to a minimum. Treatment withdrawal is therefore best done in theoperating theatre complex wherever possible, although there may be local constraints and family preferences that prevent this.
Three contingencies need to be considered:
- A clinician (or appropriately trained and certified nurse practitioner) must be readily available throughout the period of withdrawal, whether it takes place in the theatre complex or in ICU, to enable prompt confirmation of death when it This clinician must not be a member of the NORS team.
- The next-of-kin must be given the opportunity to be present before and during treatment withdrawal and until death is confirmed. Their privacy must be respected; a room should be available into which they may withdraw at the time of death and where they may remain undisturbed for a period of time following death.
- Some potential donors do not die in a manner or time frame conducive to successful organ If the potential donor remains alive beyond the period of time that the retrievalsurgeons deem appropriate (a minimum of 3 hours), it is not appropriate to keep them in the operating department. Since death is inevitable, the patient must be transferred to the most appropriate alternative location, which may be a bed/single room in an adjacent ward or transferred back to the ICU. This possibility must be explained to the next of kin at the timedonation is discussed.
6.1.3 Immediately Before Treatment Withdrawal
Before withdrawal of treatment, the following preparations apply:
- Retrieval teams for all the organs must be present and prepared in theatre. Adequate numbers ofnon-scrubbed staff must be available to allow for rapid transfer of the donor from bed to the operating table.
- The preservation fluid must be run through the giving sets, although the bags themselves must remain on ice. Heparin should be added to the first 2 litres of preservation solution to be perfusedthrough the aorta and portal vein at a dose of 300 IU/kg donor body weight (around 25,000 IU for an 80kg person).
- While it may be desirable to administer heparin at the time of treatment withdrawal or unexpected arrest, as recommended by the American Society of Transplant Surgeons (2), this is not permitted in Maastricht type 1, 2, 3, or 5 DCD donors in the UK by the Human Tissue Act 2004 and the Human Tissue (Scotland) Act 2006 (3,4). Prior cannulation in Maastricht category 3controlled donors is also currently
- If the DCD donor is Maastricht type 4 and death has previously been certified by brain stemcriteria, the donor can be heparinised with 300 IU/kg (approximately 25,000 IU for an 80kg person) immediately before treatment withdrawal.
6.1.4 Mode of Withdrawal of Life-Sustaining Treatment
Following a diagnosis of futility, the supervising clinician will withdraw life-sustaining treatment. Typically, this includes withdrawal of ventilatory support and vasopressor infusions but may also involve withdrawal of extracorporeal membrane oxygenation (ECMO) support.
To ensure the best transplant outcome from the donated organs, abrupt cessation of ventilation, extubation, and discontinuation of any vasopressor drugs induces the shortest withdrawal time andassociated warm ischaemia to the donated organs.
The retrieval teams must be aware of how treatment withdrawal will be performed. The manner of treatment withdrawal is for the supervising intensivists to decide and must not be dictated by the NORS or transplant teams. Timing of retrieval will be arranged between the SNOD, the respective NORS team lead and the recipient centre(s) in collaboration. Organ Donation and Transplantation Hub Operations will keep the NORS team and the transplanting centre informed of any delays at recipient centres. Any changes to agreed retrieval times must therefore be escalated to Hub Operations.
6.1.5 After Treatment Withdrawal
Following treatment withdrawal, regular contact will be maintained with the SNOD regarding blood pressure and arterial saturations on the donor. The SNOD will record serial haemodynamic measurements every 5minutes, including heart rate, blood pressure, arterial oxygen saturations, respiratory rate, and regular bloodgases if available.
6.1.6 The Relevance of Time from WLST to Death
Various factors are known to predict the likelihood of death after withdrawal of life supporting treatment (6-8), but none are definitive. The haemodynamic changes following treatment withdrawal vary, with some patients showing an immediate fall in blood pressure while others have a sustained good blood pressure for minutes or hours before it eventually falls (9). The donor agonal phase therefore remains highly unpredictable and the effects on organ transplantation outcomes are likely multi-factorial, including incremental warm ischaemic insults and tolerance for warm ischaemic injury. Some authors suggest that organs are not removed after certain time periods after WLST due to risk of graft failure (6,10,11).
Due to logistical considerations, and as a standard, following WLST from a potential controlled DCD donor, cardiothoracic NORS teams wait for a minimum of two hours, while abdominal teams wait at least three hours. The ‘stand down’ process for individual organs can further be decided and adjusted in conjunction with recipient centres. Liver and pancreas recipient centres traditionally stood down on the retrieval if asystole did not occur within 30 to 60 minutes following WLST. However, introduction of new techniques for in situ and ex situ perfusion and viability testing, or case-by-case considerations, may extend this period. Cardiothoracic teams are advised to have a discussion with the recipient centre for the heart after one hour. For kidneys, a minimum of three hours waiting applies, irrespective of haemodynamic parameters of the donor. If the donor fails to proceed to asystole within the three hours and the haemodynamic parameters are not showing a decline towards a predictable asystole, the abdominal NORS team generally can stand down on the retrieval after discussion with the recipient centre.
6.1.7 Functional Warm Ischaemia Time
The most important consideration during the withdrawal phase is the perfusion of the organs; a prolonged withdrawal phase is unlikely to be harmful as long as the blood pressure is maintained and urine output continues. To address this, a threshold systolic blood pressure of 50 mmHg has been proposed below which warm ischaemia injury occurs (12). This has been adopted widely so that the functional warm ischaemia period (FWIT) starts when the systolic blood pressure has had asustained (at least two minutes) fall below 50 mmHg and extends up to the onset of cold in situperfusion or commencement of NRP (see also Chapter 3).
The FWIT reflects the fact that, even though a circulation exists, end-organ perfusion is poor and the organs suffer a warm ischaemic insult. It may be appropriate, therefore, to consider this warm ischaemic period when assessing likely organ damage, rather than solely the asystolic warm ischaemic period.
Evidence for the threshold blood pressure is limited, particularly for liver transplantation, and different blood pressure thresholds may be appropriate. The threshold may vary with age and baseline blood pressure, such that a hypertensive 60-year-old will suffer ischaemic damage when the systolic pressure is much higher than 50 mmHg, in contrast to a young normotensive patient. The implanting surgeon needs to take all these factors into account when evaluating the viability of the donatedorgans. Donor, graft, and recipient characteristics, as well as preservation methods should be considered in the final decision about organ utilisation.
Pulse oximetry, while being used to inform the onset of organ hypoxia, when measured with a peripherally placed pulse oximeter will only reflect peripheral hypoxaemia. It is common for donors to become peripherally shut down as the process of dying proceeds, which reduces the accuracy of oximetry. For this reason, oxygen saturation is no longer part of the definition of FWIT in the UK but may be used by some but without a solid evidence base.
The quality of the hemodynamic measurements during the withdrawal phase should be taken into account and, when possible, obtained at the most accurate level. Non-invasive measurements of SpO2 by pulse oximetry become inaccurate when SpO2 drops below 80% and should preferentially be replaced by blood gas measurements if available. Non-invasive cuff measurements of blood pressure are generally more inaccurate, while arterial line provides continuous assessment.
Once the systolic BP has fallen below 50mmHg (i.e., onset of FWIT), most of the recipient centres would accept a 30 minutes time interval until cold perfusion for liver and pancreas, one hour before abandoning the lungs, and three hours before abandoning the kidneys as deemed untransplantable due to excessive warm ischaemia. Evidence supporting these thresholds are lacking but they are endorsed by NHSBT retrieval protocols and practiced by NORS teams.
6.1.8 No-touch Period and Declaration of Death
The no-touch period starts when cardio-respiratory arrest is confirmed. This is an additional period of stand-off before declaration of death is confirmed and laparotomy and organ perfusion with cooled preservation solution is allowed to commence. This brief period varies between countries and legislations from two to 20 minutes. In the UK, death is confirmed after 5 minutes of continuous absence of cardio-respiratory function, without any evidence of resumption of circulation (5). There is no need for any further stand-off period once death is verified.
6.1.9 Controlled DCD
Once treatment is withdrawn in Maastricht type 4 DCD donors there is no need to verify death: death has already been declared. It has been agreed that a no-touch period of five minutes following circulatory arrest be respected before a Maastricht type 4 DCD donor is transferred to the operating room and retrieval procedure commences.
6.1.10 Continued Cardiopulmonary Resuscitation or Re-commencement of Cardiac Contractility
Additional precautions may be necessary should interventions risk restoring the supply of oxygenated blood to the brain (see chapter 7 on NRP). Also, should the heart re-commence functional contractility during retrieval surgery, the team should immediately abandon all retrieval-related interventions, including any form of ventilation, and stand away from the donor. Following this the NORS Lead Surgeon should follow NORS guidance, which includes contacting the donor hospital’s anaesthetic/critical care teams to reinstate monitoring and consider the provision of analgesic or sedative agents if indicated.
6.2 Steps of DCD Abdominal Organ Retrieval
Individual techniques for organ retrieval vary. The principle is to rapidly cool the organs by acombination of intra-arterial perfusion with ice-cold preservation solution and application of topical slush. Once cold, the organs need to be removed quickly but with care to ensure no inadvertentdamage, particularly to anomalous vessels.
Once death is confirmed:
- The patient is transferred to the operating table.
- The standard retrieval procedure derives from the super-rapid technique.
- The chest and abdomen are rapidly cleaned with antiseptic and drapes are applied.
- A midline incision is made from the suprasternal notch to the symphysis pubis. The abdomen and chest are opened.
- The liver should be protected with a swab during median sternotomy and, during mobilisation; it should be retracted gently to prevent avulsion of its peritoneal attachments.
- The abdomen is kept open using a large self-retaining retractor handed half open (for speed of action).
- The distal ileum, caecum and small bowel are reflected superiorly, exposing the area of the aorto–iliac bifurcation sufficient to rapidly identify and cannulate the distal aorta or the right common iliac artery.
- Cold perfusion is started immediately by gravity using an approved cold preservation solution containing 300 IU/kg of heparin. The IVC must be vented early on to prevent congestion of the liver. This can be via the abdominal IVC or more preferably the right atrium; easily performed by opening the diaphragm or with a thoracotomy.
- Venting the abdominal IVC is preferable if the lungs are also being retrieved. This is done by cannulating the inferior vena cava just above the confluence of the common iliac veins to allow the blood to siphon out to a receptacle.
- A sternotomy using a Gigli or automated sternal saw can then start and a Finocchietto retractor can be placed. The right atrium can be partially divided to improve venous venting and both pleura are opened to facilitate drainage into the pleural cavities where two pool suction tubes are placed to collect the effluent blood and perfusion solution. The left lung is lifted exposing the descending thoracic aorta and at this time a cross-clamp can be applied.
- Following aortic cross-clamping, a pressure cuff can be applied to the perfusion bag to improve perfusion pressure in the aorta.
- Ice slush is then placed across the liver, between the bowel loops, in the lesser sac, in front of each kidney, and in the right hemi-thorax on the dome of the diaphragm above the liver. The common bile duct is then ligated distally and divided and the proximal duct is flushed with preservation solution, and the gallbladder fundus incised and bile washed out.
- If the liver is being retrieved, the portal vein needs to be directly isolated after division of the common bile duct and cannulated approximately 1cm above the duodenum. When the pancreas is not being retrieved the superior or inferior mesenteric veins may be cannulated.
- Dissection should now wait until the organs are properly perfused and cooled. Allow at least two litres of UW preservation fluid through the organs before starting final dissection to remove them, more if HTK is being used.
- The first two litres of cold preservation solution that is flushed through the aorta and first litre through the portal vein should each contain 300 IU/kg of heparin. Usually, the flow of preservation solution in the portal vein can be slowed down after 1 litre of in situ perfusion with UW, more if HTK is used.
- The liver is retrieved first, followed by the pancreas and kidneys.
- One option is to retrieve the liver and pancreas en bloc and subsequently separate the two organs on the back bench. Although there is no clear advantage for this, it remains the preference of some NORS teams and is particularly suitable if both organs are returning to the same implanting centre since they can be split there, and not at the donor hospital.
Caveats:
- Cannulating the common iliac artery may prejudice its use for reconstructing the arterial supply to the So, if it is used, divide it as close to the aortic bifurcation as possible.
- Neither the superior nor inferior mesenteric veins should be cannulated when the pancreas is being retrieved, since the venous perfusion pressure opposes the arterial perfusion pressure in the pancreas and prevents cellular perfusion.
- Since establishing dual as opposed to aorta-only preservation is not innocuous in the context of DCD and can prolong donor warm ischemia, it may be preferable to perform additional portal preservation subsequent to the onset of aortic preservation, either in situ in the donor or on the back table.
6.2.1 Removal of the Liver
- Liver retrieval is continued as in the cold phase of DBD retrieval (at a faster pace, as in an unstable DBD).
- Gastro-hepatic omentum is divided close to lesser curve of stomach to protect any aberrant left hepatic vessels.
- Greater omentum is divided close to greater curve all the way up to the cardio-oesophageal junction.
- Stomach is divided proximal to pylorus with a stapler and the stomach can be pushed up into the chest to keep it away from the operative field.
- After completion of perfusion, divide the gastro-duodenal artery and divide the portal vein. Ensure that there is no aberrant right hepatic artery on the right side of gastro-duodenal ligament.
- If an aberrant right hepatic artery is present, it should be removed with a segment of SMA without damaging the pancreas (in pancreas retrievals). If there is no pancreas retrieval, it is safer to remove the liver with the head of the pancreas to protect this artery.
- Infrahepatic IVC is divided above the level of renal veins.
- Intrapericardial IVC is divided.
- Coeliac axis dissection is completed by dividing the splenic artery and creating an aortic patch at the origin.
- Liver is removed with attached diaphragm postero-superiorly.
- Donor hepatectomy time, defined as time from flush to liver out of the body, should be kept as short as possible – at most 60 minutes from the start of cold perfusion. This should be balanced against the risk of injuries.
6.2.2 Removal of the Pancreas
- The pancreas can be removed en bloc with the liver, or separately from the liver. Removal en blochas the advantage of allowing identification of accessory or replaced right hepatic arteries arising from the superior mesenteric artery (SMA).
- En bloc retrieval of liver and pancreas is accepted, but undue delay and relative warm ischaemia whilst splitting organs on the back table is a risk in this case and must be avoided. Nonetheless, the speed of en bloc retrieval in DCD donors reduces time to place organs on ice, which is a desirable outcome.
- Handling of the pancreas should be kept to a minimum; during mobilisation the spleen should be used as a handle.
- The duodenum is Kocherised. The gastro-colic ligament is divided fully to expose the body and tail of the pancreas.
- The short gastric vessels are divided close to the stomach and the tail of the pancreas is mobilized by using the spleen as a handle to lift it medially.
- The transverse mesocolon is divided near the colon and the small bowel divided just beyond the duodenojejunal flexure using a linear stapler; the stomach is similarly divided just before the pylorus.
- The small bowel mesentery is then stapled, staying away from the pancreas in order to avoid damage to the inferior pancreatico-duodenal artery.
- If the pancreas is to be removed en bloc with the liver it is next mobilised; if it is being removed afterthe liver has been removed the tail of the pancreas should now be lifted via the spleen and theunder surface of the gland dissected away from the underlying Gerota’s fascia.
- The SMA is divided at the level of the aorta without an aortic patch to avoid damage to the aorticpatch that is required for the kidneys.
6.2.3 Removal of Kidneys
- The right colon is reflected upwards to expose the inferior vena cava (IVC), aorta, ureters and Gerota’s fascia around the kidneys.
- If pancreas is not being retrieved, Kocherisation would expose the left renal vein.
- The ureters are divided as distal as possible and a marker clip placed on the end of Sufficient soft tissue is included to preserve ureteric blood supply. Care must be taken not to exert undue traction on the renal pedicle.
- The left renal vein is divided at its confluence with the IVC leaving the IVC intact to go with the right kidney; the cut end of the left renal vein is then reflected laterally.
- The anterior wall of the aorta is incised in the midline through to the origin of the SMA. The posteriorwall is similarly incised between the origins of the lumbar A generous amount of aorta plus underlying tissue is removed with the renal arteries; no attempt should be made to identify the arteries at this stage, although their ostia should be identified from within and care taken to ensurethat they are on the patch.
- The kidneys in their pocket of perinephric fat are then held one by one and dissection continued posteriorly onto psoas to avoid damage to the arteries. Avoid pulling the kidney away from the body – intimal arterial tears are readily made, particularly in older donors.
- Alternatively, en bloc removal of both kidneys may be performed. Both ureters are divided as they cross the iliac artery and their ends clipped and held up. The distal aorta and IVC are also held up by clips and the tissue behind the aorta, cava and ureters is divided progressively more Both kidneys come out as a bloc.
- The en bloc technique iis preferred for retrieval of small paediatric kidneys (see 6.7 below) and may be appropriate if both kidneys are known to be going to the same recipient centre, or in rare cases of horseshoe kidney. This technique is associated with less damage to the kidneys.
6.2.4 Removal of Additional Blood Vessels
- Liver and pancreas retrieval must always be accompanied by the blood vessels needed for reconstruction in the recipient.
- A good quality iliac artery with common, internal and external iliac arteries should accompany the pancreas for arterial reconstruction.
- The iliac arteries and veins should not be “pulled” during removal. Unsuspected arterial dissection as a consequence may have grave implications for a liver or pancreas recipient.
- Following removal of the organs, an extensive evaluation must be made of the abdominal and thoracic contents for evidence of cancer, in particular of the colon, stomach, pancreas, oesophagus and DCD donors require exactly the same scrutiny as DBD donors.
- It may only be possible to examine the viscera in the donor once the organs have been retrieved in the DCD setting. Nonetheless, the same detailed scrutiny must be applied.
6.2.5 Back Table Preparation
- As the organs are removed, they are best placed in bowls containing saline slush to aid cooling.
- Back table liver perfusion should be carried out through hepatic artery, portal vein, and the bile duct.
- The kidneys should also be exposed by removal of the perinephric fat before cold storage, looking for tumours and checking perfusion. Any abnormal lesions should be removed and urgent histology obtained before any of the organs are implanted.
- If perfusion is poor, the renal artery/ies may be further perfused with preservation solution.
6.2.6 Organ Damage
If damage has occurred this must be recorded on the relevant NHSBT form and the recipient surgeon should be notified. No attempt should be made to repair the damage at the donor hospital; this is bestdone by the implanting surgeon. NORS teams are encouraged to ask SNODs to document damage with appropriately taken photographs.
6.2.7 Packing for Cold Storage
Following cold in situ perfusion, the organs are still relatively warm and must be packed in ice as soon as possible. It is best to avoid prolonged back table dissection in the donor hospital. If the pancreas and liver are removed en bloc, and recipients are at different centres, it is required to split the bloc at the donor hospital; care should be taken to keep both organs cold during this and avoid inadvertent rewarming in air. If the liver and pancreas are allocated to the same centre, it is acceptable to separate the organs at the recipient centre.
6.3 Steps of DCD Lung Retrieval
The donor ICU team
-
The retrieval team and the SNOD are not involved in any way with the management of the donor priorto retrieval, so there is a reliance on the donor ICU team.
-
Ventilator management should be with a lung-protective regimen, 5-6 mL/kg tidal volume and PEEP of8 cmH2O.
-
In an ideal situation, there will have been a bronchoscopy, with a report of the state of airway mucosa and secretions, recent ABGs and a chest X-ray within 12 hours.
-
A nasogastric tube should be placed unless there is likelihood of distress to the donor.
-
The situation with regard to re-intubation by the donor hospital team must be ascertained before the retrieval team leave their base. If the donor hospital team will not re-intubate the donor, due to concerns about a potential conflict of interest, the retrieval team must include someone appropriately skilled.
The retrieval team
-
The local arrangements should be confirmed with the SNOD with regards to personnel to re-intubate. Potential difficulties with intubation should be identified at this stage.
-
A discussion should take place with the abdominal retrieval team with regard to how the surgery should be planned. If possible, discussions should also be held with the donor team about the use of the operating theatre anaesthetic machine to deliver continuous PEEP.
-
After withdrawal of treatment, regular contact should be maintained with the SNOD with regard to the haemodynamics and oxygen saturation of the donor. If cardiac arrest has not occurred within 60 minutes, the situation should be discussed with the implanting team. If the donor condition remains stable, a decision may be made to abandon the retrieval at this stage.
After circulatory arrest
Note: It has been agreed that the lungs will not be re-inflated until 10 minutes has elapsed sincethe time of death, to avoid any chance of auto-resuscitation. Similarly, cyclical ventilation should not be used until the chest is open and there is no longer any chance of brain stem activity; isolation ofthe cerebral circulation is recommended.
-
On arrival in the operating theatre, the donor should be re-intubated with a cuffedendotracheal tube; this may be preceded by a rigid bronchoscopy, depending on the skills available.
-
Intra-abdominal manipulation may cause aspiration, so early protection of the airway is important.
-
Thorough airway toilet should be performed as soon as possible.
-
Once reintubated, atelectatic lung may be recruited with a single breath, perhaps 25 mmHg pressure for 40 seconds, ideally using the anaesthetic machine. CPAP should be maintained at 5 cmH2O and continuous O2, once again possibly with the theatre anaestheticmachine or an appropriate valve.
-
Cyclical ventilation must not be used until the chest is open and the aorta can be clamped. The time of lung inflation should be noted. Warm ischaemia is lessened at this stage, but many teams feel that early flushing of the lungs is still important, so the chest is rapidlyopened.
-
The lungs should be examined for collapse, consolidation, mass lesions and pleuraladhesions. If there is a suspicion of airways disease, the degree of collapse when the lungs are disconnected should be noted. This test should be done before flushing.
-
The pulmonary artery should be cannulated, and the right ventricle may be opened to removeclot.
-
Antegrade perfusion should be started as per the practice of the retrieval team. Many will begin with warm Perfadex®. The left atrium or atrial appendage should be widely opened and clot washed out of the pulmonary veins. At this stage, distribution of perfusate is aided by gentle cyclical or intermittent recruitment and ventilation of the lungs.
-
When the antegrade perfusion is complete, the pulmonary veins should be gently cannulated for retrograde perfusion, 200-500 mL down each one, until the effluent from the pulmonary artery is clear. The lungs may be removed collapsed, although some retrieval teams may continue to ventilate. If they have been collapsed when only the trachea is intact, the lungs should be cautiously re-inflated prior to storage. After removal, the lungs should again beexamined.
-
Information about the degree of inflation, the “collapse” test, any areas of consolidation or masses, clot in the pulmonary artery, the uniformity of flushing, and any palpable oedema will be required by the implanting team. They will decide to use the lungs outright, to place them on ex vivo lung perfusion (EVLP, also called ex situ normothermic lung perfusion), or to abandon the retrieval.
-
Communication between retrieving and implanting team must be regular and complete. Thedecision about use of the lungs will usually require a detailed discussion between retrievingand implanting surgeon.
-
If EVLP is to be used, at least 10 cm of trachea and ample LA and PA should be retrieved if possible. The lungs should be packed as for a standard retrieval, with the routine bloodspecimens and paperwork.
6.4 Steps of DCD Heart Retrieval
DCD heart retrieval is performed with ex situ machine perfusion (chapter 14). The heart is otherwise excised in the standard fashion for heart retrieval.
6.5 Preservation Solutions
The choice of organ preservation solution should be determined by guidance from NHSBT. Preservation solutions are further discussed in organ-specific chapters (see Chapters 9-14).
6.6 Heparin and Fibrinolytics
Heparin is added to prevent clots from forming once the preservation solution is infused; the preservation solution alone will cause clot formation. Some units consider the addition of athrombolytic agent based on evidence from animal and small clinical trials (13-17). The effectiveness of agents such as streptokinase in the hypothermic environment is questionable,although some authors have suggested an initial warm ‘pre-flush’ to overcome this problem.
6.7 Paediatric Retrieval
Smaller cannulas may be needed. En bloc removal is the preferred technique for retrieval of small paediatric kidneys (aged under 5 years old). In small paediatric kidney retrieval (donor weight <20kg), a cuff of bladder should be provided with each ureter to facilitate implantation.
6.8 Staffing the Retrieval Procedure
The ‘stand alone’ National Organ Retrieval Service (NORS) teams are responsible for retrieving organs from both DBD donors and controlled DCD donors. Because of the severe time limitations, uncontrolled donors are outside the remit of the NORS teams.
Currently there are ten abdominal and six cardiothoracic on-call NORS retrieval teams, which cover the whole of the UK. These teams are commissioned and fully funded by NHSBT. Each team includes a lead surgeon, an assistant surgeon and a scrub nurse. All the cardiothoracic and four of the abdominal teams also include a perfusionist. In addition, in some regions, two SNODs attend a DCD donor, one of whom accompanies the donor family and liaises with the donor hospital staff whilst the other SNOD accompanies the retrieval team and assists with perfusion if required.
In general, the closest available NORS team should attend the donor. If there is doubt about the suitability of a particular DCD organ for transplant, and especially in small paediatric donors, the recipient centre may send an individual to join the designated retrieval team and assess the organ in situ provided that this does not delay the retrieval procedure.
References
- Organs for Transplants: a report from the Organ Donation Taskforce, January 2008. Accessed at http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_082120.pdf
- Reich DJ, Mulligan DC, Abt PL, et al. ASTS recommended practice guidelines for controlled donation after cardiac death organ procurement and transplantation. Am J Transplant2009;9:2004-11.
- Human Tissue Act London: HMSO; 2011. Accessed at www.hta.gov.uk/legislationpoliciesandcodesofpractice/legislation/humantissueact.cfm
- Human Tissue (Scotland) Act 2006. 2011. Edinburgh: Scottish Government. Accessed at http://www.legislation.gov.uk/asp/2006/4/contents
- Simpson A Code of Practice for the Diagnosis and Confirmation of Death. London, UK: Academy of Medical Royal Colleges, 2008. Accessed at http://www.bts.org.uk/MBR/Clinical/Publications/Member/Clinical/Publications.aspx?hkey=0bca99a9-40c2-4d40-bb9f-510e30d769b9
- Suntharalingam C, Sharples L, Dudley C, et al. Time to cardiac death after withdrawal of life-sustaining treatment in potential organ donors. Am J Transplant 2009;9:2157-65.
- DeVita MA, Brooks MM, Zawistowski C, et al. Donors after cardiac death: validation of identification criteria (DVIC) study for predictors of rapid Am J Transplant 2008;8:432-41.
- Lewis J, Peltier J, Nelson H, et Development of the University of Wisconsin Donation After Cardiac Death Evaluation Tool. Prog Transplant 2003;13:265-73.
- Levvey BJ, Westall GP, Kotsimbos T, et al. Definitions of warm ischemic time when usingcontrolled donation after cardiac death lung Transplantation 2008;86:1702-6.
- Bernat JL, D’Alessandro AM, Port FK, et Report of a national conference on donation aftercardiac death. Am J Transplant 2006;6:281-91.
- Reid AW, Harper S, Jackson CH, et al. Expansion of the kidney donor pool by using cardiac death donors with prolonged time to cardiorespiratory arrest. Am J Transplant 2011;11:995-1005.
- Ho KJ, Owens CD, Johnson SR, et al. Donor postextubation hypotension and age correlate withoutcome after donation after cardiac death Transplantation 2008;85:1588-94.
- Minor T, Hachenberg A, Tolba R, et al. Fibrinolytic preflush upon liver retrieval from non-heartbeating donors to enhance postpreservation viability and energetic recovery upon Transplantation 2001;71:1792-6.
- Gok MA, Shenton BK, Peaston R, et al. Improving the quality of kidneys from non-heart-beating donors using streptokinase: an animal model. Transplantation 2002;73:1869-74.
- Favreau F, Thuillier R, Cau J, et al. Anti-thrombin therapy during warm ischemia and coldpreservation prevents chronic kidney graft fibrosis in a DCD Am J Transplant 2010;10:30-9.
- Gok MA, Shenton BK, Buckley PE, et al. How to improve the quality of kidneys from non-heart-beating donors: a randomised controlled trial of thrombolysis in non-heart- beating Transplantation 2003;76:1714-9.
- Hashimoto K, Eghtesad B, Gunasekaran G, et al. Use of tissue plasminogen activator in livertransplantation from donation after cardiac death Am J Transplant 2010;10:2665-72.
7. Normothermic Regional Perfusion
We recommend that:
- Abdominal normothermic regional perfusion should be used in all DCD retrievals where there is a trained team competent to perform it. (1B)
- In the absence of other contraindications, if the two-hour ALT is under 500 iu/L and there is a glucose rise at the start of NRP, the liver should be considered suitable for transplant. Beyond that, further ex situassessment may be necessary. (1C)
We suggest that:
- Femoral cannulation is preferred if a thoracic retrieval team is present. (2C)
- The duration of the period between withdrawal of life-sustaining treatment and asystole is not viewed as a contraindication to utilising a liver from a DCD donor that is recovered using NRP. (2C)
- The use of thoraco-abdominal NRP should be further evaluated. (2D)
7.1 Introduction
Normothermic regional perfusion (NRP) is a technique developed in Barcelona by Juan-Carlos Garcia-Valdecassas, Ricardo Valero and Constantino Fondevila for uncontrolled DCD donation of kidneys (1) and livers (2), and pursued in France for the same indication (3). It had also been used in a single centre in the US(4). It involves connecting the donor to an extracorporeal circuit after death to pump the donor’s blood from the vena cava through a heater and oxygenator and back into the aorta, either directly or via iliac or femoral vessels. In doing so, a blood supply to the abdominal organs is restored.
In the early literature, NRP is referred to as normothermic recirculation, normothermic extracorporeal membrane oxygenation (ECMO), and abdominal normothermic oxygenated recirculation (3). The term normothermic regional perfusion was coined by Paul Murphy, an intensivist from Leeds working for NHSBT, to stress that the perfusion should not involve perfusion of the brain in accordance with the Association of Medical Royal Colleges Code of Practice for the Diagnosis of Death (5).
NRP was developed in the UK following a demonstration by Stephen Large in 2006 in Cambridge which showed that restoring circulation of oxygenated blood to a controlled DCD donor after a circulatory arrest could result in sustained and effective contraction of the heart (6), an observation that prompted the development of DCD heart donation internationally. At the same time, the liver and kidneys resumed their normal colour and function, and the bowel began peristalsis. It is noteworthy that Kumud Dhital, who is recorded as performing the world’s first DCD heart transplant series in the modern era (7), was Stephen Large’s assistant during that first NRP in Cambridge. This was the first case of NRP being used in controlled DCD donation.
Following that case, separate programmes were established at Papworth and in Cambridge evaluating cardiac and abdominal NRP (8,9) respectively, followed by the initiation of a programme in Edinburgh (10).
At the time of writing (2023) the technique is commonly performed in controlled DCD donors in Spain and is mandatory for DCD liver recovery in France, Italy, and Norway (11-15). It has also been taken up by centres in the Netherlands, Belgium, China and the United States, often in the context of thoracoabdominal NRP for heart retrieval (16-18).
7.2 Mechanism of Action
There are now many (non-randomised) reports showing that NRP results in greater utilisation and superior outcomes for livers and kidneys from DCD donors (11,13,19-21). It is not completely clear why NRP has proved so beneficial for DCD donors, but it has been shown that a period of in situ perfusion after warm ischaemia allows replenishment of ATP stores (22). In addition, it is likely that there is an ischaemia-preconditioning effect once the circulation is restored (23), allowing better tolerance of subsequent periods of cold ischaemia.
7.3 Clinical Procedure of NRP
7.3.1 The Perfusion Device
The first requirement of NRP is a machine to pump the blood around the circuit. There is a choice between a dedicated ECMO device, such as the Maquet CardioHelp (Gettinge, Germany), or a specially designed NRP device such as the Donor Assist (XVIVO, The Netherlands). The choice of device will dictate the circuit components and disposable costs. Current devices require a lot of assemblies and it is hoped that a simpler device which permits easy and rapid circuit assembly will be developed soon.
7.3.2 The Circuit
In the UK at present the NRP circuit is an open circuit, that is, it comprises gravity drainage into a hard-shell reservoir which is open to air. This reservoir drains into a pump which drives blood through an oxygenator which warms and oxygenates the blood before being returned to the patient. The Donor Assist comes with a pre-specified circuit; the Maquet Cardiohelp uses a bespoke circuit which at the time of writing is standardised in the UK.
At the time of writing the circuits contain a leucocyte filter to try to remove circulating white cells and minimise their effect on reperfusion injury. This is not widely used overseas, and the long-term availability of a leucocyte filter is in question so it is likely this component will be removed in the future.
7.3.3 The Perfusate
The prime solution
The circuit is primed before connection to the donor so that it contains fluid to prevent airlocks. This prime solution contains compound sodium lactate (Hartmann’s) solution, together with the following components:
- Heparin: 50,000 units to prevent thrombosis of the unheparinised donor blood as it mixes with the prime and circulates.
- Sodium bicarbonate: 1 mmol/kg body weight (1 mmol = 1 mL of 8.4% solution) to counter the acidosis that results from the asystolic period and lactate accumulation.
- Antibiotics: to prevent infection. The mucosal integrity to microorganisms is breached during asystole and translocation into the systemic circulation may occur, hence the need to ensure no contamination of the organs being retrieved.
- Phentolamine: the period up to death is characterised by massive catecholamine release in the donor which continues early after reperfusion. Phentolamine is an alpha-blocker that counters the alpha-adrenergic vasoconstriction.
- Methylprednisolone: is administered to brain-dead donors and probably has a role in NRP DCD donors to reduce reperfusion injury.
The evidence as to the efficacy of some of these additives in the NRP setting is limited or absent and merits future investigation. The composition of the prime varies widely between countries, with many centres having additional mannitol (acting as a free radical scavenger) and others using Plasmalyte in place of Hartmann’s. The use and choice of antimicrobials also vary.
Anaemic or small donors
Where the donor is anaemic, or if the donor has a small circulating volume (e.g., they are a child or small adult) then it is prudent to substitute some of the Hartmann’s solution in the prime with packed red blood cells to maintain a haemoglobin concentration above 80 g/L.
Additional fluids during retrieval
Since the perfusate lactate concentration is used as a readout of function, it is important not to add Hartmann’s solution to the perfusate once perfusion has begun, since Hartmann’s contains 29 mmol/L lactate. Instead, it is recommended to use Gelofusine or blood.
If there is a lot of bleeding during NRP then paradoxically it is important to add more heparin, since the fluid used to replace losses will not contain it and the heparin concentration will be reduced. Regular monitoring of the activated clotting time will also help but is generally not necessary in the first two hours.
7.3.4 Cannulation
Cannulation of the aorta and vena cava or femoral vessels
NRP requires the placement of large cannulae on both venous and arterial sides of the circulation. Typically, this can either be in the femoral artery and vein, or more commonly (and more simply, for new teams) in the aorta or common iliac artery, and the IVC or common iliac vein. While placing a venous cannula first allows rapid decompression of the congested viscera, spillage of blood at this stage can make planes difficult to identify and complicate subsequent arterial cannulation. Therefore, it is recommended to first place the arterial cannula and then rapidly place the venous cannula.
Cannulation follows the same techniques used in cold perfusion, with control of the distal vessel with either a ligature or a clamp, and clamping of the proximal part of the vessel to temporarily prevent blood leaking until the vessel is opened and the cannula passed in. The cannulas need to be of a suitable size to achieve flows up to 5 L/min.
Cannulation of the ascending aorta
In order to ensure there is no brain perfusion, it is mandatory to place a large cannula in the ascending aorta open to air so that the pressure in the aorta can be monitored. Typically, there is a 2 to 5 cm column of deoxygenated blood, demonstrating that there is no pressure and thus no perfusion of the brain (24).
7.3.5 Haemodynamic Goals
Once the circuit is established, perfusion should start immediately. The ascending aortic cannula is placed and the descending thoracic aorta is occluded, either by a clamp or, in Scotland, an Endoclamp. The aim is to achieve flows to the abdominal organs of over 2.5 L/minute; if the legs are in the circuit and being perfused, or the donor is large, higher flow rates are required.
7.3.6 Monitoring During NRP
Throughout the period of NRP, it is important to ensure adequate oxygenation and monitor the function of the liver. Urine output usually ceases during NRP and is not thought to reflect post-transplant renal function.
Blood gases
Adequate oxygenation and maintenance of pH are achieved by alteration of the gas flow across the oxygenator and the composition of that gas. Oxygenation is adjusted to keep the venous haemoglobin oxygen saturation (SvO2) between 60% and 80%, while the pH is adjusted by reference to the arterial pH. It is uncommon to have to supplement the perfusate with bicarbonate since a functioning liver should maintain pH without intervention.
Liver assessment
Liver function during NRP is assessed by looking at three biochemical parameters: glucose, lactate, and the release of alanine transaminase (ALT). At the start of NRP, the glucose is raised (a response to adrenaline during withdrawal and ischaemia) and the lactate will be high. Lactate will subsequently fall during NRP, but unlike ex situ perfusion, it will not fall to normal due to lactate-rich blood coming back from the limbs. The lactate fall is greater when a clamp is placed across the SVC preventing upper limb blood return.
ALT is released from the liver upon reperfusion. Following an initial enzyme release, the concentration usually plateaus. Therefore, a perfusate sample should be measured soon after starting NRP, and again at intervals afterwards (60 and 120 minutes). It may be helpful to take a measurement at 30-minute intervals, so there will be a series of readings if the NRP has to be stopped prematurely for some reason.
In the early work on NRP using uncontrolled donors, Fondevila et al used a threshold of 4 times the upper limit of normal for transaminase values, below which they were happy to use the liver (2). At the time of writing the authors are content to use a rise of less than 10 times the upper limit of normal (~500 IU/L), providing the concentration has plateaued between the first and second hour. Livers in the range 10 to 20x the upper limit of normal (500-1000 IU/L) then undergo ex situ normothermic perfusion to assess further before a decision to use or not use is made.
There is no evidence to support limiting organ utilisation based on the duration of the withdrawal period when NRP is used, with reports of livers being used successfully three hours after withdrawal of life-supporting treatment, with good long-term outcomes (25). Recent data from Spain also suggest that long cold ischaemic times and the use of NRP livers for re-transplant are associated with less good outcomes, but confirm that DCD donor livers recovered using NRP, experience a very small incidence of biliary complications (25).
7.3.7 Staffing the Retrieval Procedure
NRP requires two key members of staff, amongst many others. The first is an advanced perfusion practitioner who runs the pump and monitors the blood gases and adjusts flows and gas delivery accordingly. The second is a surgeon trained in establishing NRP in the donor. This not only includes cannulation, but also placing the thoracic occlusion clamp and ascending aortic vent.
Once the NRP circulation is established, initial dissection should be limited to dividing the common bile duct to allow free drainage. Thereafter, it is sensible to take a break for 60 minutes or so while the organs recover from warm ischaemia before preparations for cold perfusion in situ and recovery into ice cold preservation solution.
7.4 Thoracic Organs: Specific Procedures
Thoracic organs can be recovered either after a period of thoraco-abdominal NRP or by cold perfusion while the abdominal organs are undergoing NRP. In either circumstance, because active dissection occurs from the outset it is common for there to be significantly more blood loss when thoracic organs are being recovered compared to when only the abdominal organs are recovered.
7.4.1 Thoraco-abdominal NRP
Thoraco-abdominal NRP is the restoration of an oxygenated circulation to the thoracic and abdominal organs. The challenge in doing this is to ensure there is no perfusion of the brain from the many collateral arteries. Early UK work was done without ensuring no brain perfusion, but rather assuming there was none. This is still the practice in many countries where thoraco-abdominal NRP is undertaken.
In order to ensure no cerebral perfusion occurs, it is necessary either to open the four great vessels, (both common carotids and subclavian arteries), either to air or, ideally, into cannulae which return any blood back into the hard-shell reservoir. This is challenging and time-consuming, and ways to return circulation to the heart while excluding cerebral perfusion are being explored.
Once thoraco-abdominal NRP is established, cardiac surgeons prefer to test the function of the heart by letting it support the circulation. This involves weaning the donor from the NRP circulation. During this process, it is common for abdominal organ perfusion to be suboptimal, either because the function of the heart is insufficient to sustain an effective circulation, or as a result of inotrope administration causing vasoconstriction of the mesenteric supply.
7.4.2 Cold Perfusion and Recovery of Thoracic Organs during Abdominal NRP
Direct procurement of the thoracic organs by flushing them in situ with cold perfusate is the most popular way to recover these organs at the time of writing. This risks major haemorrhage and failure of abdominal NRP since large vessels are divided (e.g., both vena cavae) and haemostasis is secondary, following safe thoracic organ recovery. The key to success is good communication with a full discussion beforehand as to what each team will do.
Lung retrieval
Following circulatory arrest and transfer into the operating room, the donor is reintubated. While the abdomen and chest are opened the lungs are insufflated with oxygen which protects the epithelium from ischaemia. They can remain in this position for up to 90 minutes, although in reality one of the thoracic team then performs a bronchoscopy to assess secretions, before re-inflating them.
In the UK it has been agreed that no lung dissection will occur for the first 30 minutes to enable the abdominal NRP to be established. Thereafter a clamp is placed across the inferior vena cava in the chest and the lungs are mobilised with careful attention to haemostasis
Heart retrieval
In the absence of thoraco-abdominal NRP, there is an imperative to remove the heart as quickly as possible. DCD hearts recovered in this way are placed on an ex situ perfusion device such as the Transmedics Heart Organ Care System (OCS) for transport to the recipient. This device requires priming with 1200 mL of blood which is recovered from the donor before abdominal NRP is started. In order to cope with this volume loss three units of packed red cells are added to the prime solution in anticipation of the blood that will be lost to perfuse the OCS machine (26).
7.5 Documentation
NHSBT has developed an organ passport to accompany the abdominal organs recovered during NRP which details the perfusate gases and biochemistry, as well as the circuit flows and all the time points from the withdrawal of treatment to packing the organs in ice. Recipient surgeons need to familiarise themselves with this and understand the clinical significance of the information that is being conveyed.
References
- Valero R, Cabrer C, Oppenheimer F, et al. Normothermic recirculation reduces primary graft dysfunction of kidneys obtained from non-heart-beating donors. Transpl Int 2000;13(4):303-10.
- Fondevila C, Hessheimer AJ, Ruiz A, et al. Liver transplant using donors after unexpected cardiac death: novel preservation protocol and acceptance criteria. Am J Transplant 2007;7(7):1849-55.
- Barrou B, Billault C, Nicolas-Robin A. The use of extracorporeal membranous oxygenation in donors after cardiac death. Curr Opin Organ Transplant 2013;18(2):148-53.
- Magliocca JF, Magee JC, Rowe SA, et al. Extracorporeal support for organ donation after cardiac death effectively expands the donor pool. J Trauma 2005;58(6):1095-101.
- Academy of Medical Royal Colleges. A Code of Practice for the Diagnosis and Confirmation of Death. London: Academy of Medical Royal Colleges; 2008: https://www.aomrc.org.uk/wp-content/uploads/2016/04/Code_Practice_Confirmation_Diagnosis_Death_1008-4.pdf.
- Ali A, White P, Dhital K, et al. Cardiac recovery in a human non-heart-beating donor after extracorporeal perfusion: source for human heart donation? J Heart Lung Transplant 2009;28(3): 290-3.
- Dhital KK, Iyer A, Connellan M, et al. Adult heart transplantation with distant procurement and ex-vivo preservation of donor hearts after circulatory death: a case series. Lancet 2015;385(9987):2585-91.
- Messer SJ, Axell RG, Colah S, et al. Functional assessment and transplantation of the donor heart after circulatory death. J Heart Lung Transplant 2016;35(12):1443-52.
- Butler AJ, Randle LV, Watson CJ. Normothermic regional perfusion for donation after circulatory death without prior heparinization. Transplantation 2014;97(12):1272-8.
- Oniscu GC, Randle LV, Muiesan P, et al. In situ normothermic regional perfusion for controlled donation after circulatory death–the United Kingdom experience. Am J Transplant 2014;14(12):2846-54.
- Hessheimer AJ, Coll E, Torres F, et al. Normothermic regional perfusion vs. super-rapid recovery in controlled donation after circulatory death liver transplantation. J Hepatol 2019;70(4):658-65.
- Savier E, Lim C, Rayar M, et al. Favorable outcomes of liver transplantation from controlled circulatory death donors using normothermic regional perfusion compared to brain death donors. Transplantation 2020;104(9):1943-51.
- Antoine C, Jasseron C, Dondero F, et al. Liver transplantation from controlled donors after circulatory death using normothermic regional perfusion: an initial French experience. Liver Transpl 2020;26(11):1516-21.
- De Carlis L, Lauterio A, De Carlis R, et al. Donation after cardiac death liver transplantation after more than 20 minutes of circulatory arrest and normothermic regional perfusion. Transplantation 2016;100(4):e21-22.
- Hagness M, Foss S, Sorensen DW, et al. Liver transplant after normothermic regional perfusion from controlled donors after circulatory death: the Norwegian experience. Transplant Proc 2019;51(2):475-8.
- Vandendriessche K, Tchana-Sato V, Ledoux D, et al. Transplantation of donor hearts after circulatory death using normothermic regional perfusion and cold storage preservation. Eur J of Cardiothorac Surg 2021;60(4):813-9.
- Ding GY, Zhao Y, Wu W, et al. In situ normothermic regional perfusion for liver donation from China category III (organ donation after brain death followed by circulatory death): a single-center cohort study. Exp Clin Transplant 2020;18(1):83-8.
- Urban M, Castleberry AW, Duncan KF, et al. Thoracoabdominal normothermic perfusion in donation after circulatory death. Ann Thorac Surg 2022;113(6):e473-e476
- Watson CJE, Hunt F, Messer S, et al. In situ normothermic perfusion of livers in controlled circulatory death donation may prevent ischemic cholangiopathy and improve graft survival. Am J Transplant 2019;19(6):1745-58.
- Ruiz P, Valdivieso A, Palomares I, et al. Similar results in liver transplantation from controlled donation after circulatory death donors with normothermic regional perfusion and donation after brain death donors: a case-matched single-center study. Liver Transpl 2021;27(12):1747-57.
- Ramirez P, Vazquez D, Rodriguez G, et al. Kidney transplants in controlled donation following circulatory death, or maastricht type III donors, with abdominal normothermic regional perfusion, optimizing functional outcomes. Transplant Direct 2021;7(8):e725.
- Garcia-Valdecasas JC, Tabet J, Valero R, et al. Liver conditioning after cardiac arrest: the use of normothermic recirculation in an experimental animal model. Transpl Int 1998;11(6):424-32.
- Net M, Valero R, Almenara R, et al. The effect of normothermic recirculation is mediated by ischemic preconditioning in NHBD liver transplantation. Am J Transplant 2005;5(10):2385-92.
- Manara A, Shemie SD, Large S, et al. Maintaining the permanence principle for death during in situ normothermic regional perfusion for donation after circulatory death organ recovery: A United Kingdom and Canadian proposal. Am J Transplant 2020;20(8):2017-25.
- Hessheimer AJ, de la Rosa G, Gastaca M, et al. Abdominal normothermic regional perfusion in controlled DCD liver transplantation: outcomes and risk factors for graft loss. Am J Transplant 2022;22(4):1169-81.
- Mohite PN, Garcia Saez D, Butler AJ, et al. Direct procurement of donor heart with normothermic regional perfusion of abdominal organs. Ann Thorac Surg 2019;108(2): 597-600.
8. Informing the Recipient
We recommend that:
- Providing information, both orally and in writing, for the potential transplant recipient is a requirement for consent and is the responsibility of the multi-disciplinary transplant team. This must be updated and reviewed annually and the outcome of discussions clearly documented in the patient’s medical record. (1B)
- Information should be tailored to the requirements of the potential recipient, recognising that not all patients wish to receive detailed information. However, this must not preclude engagement with the transplant process. (1B)
- The final risk: benefit analysis presented to the potential transplant recipient following an organ offer must explain the relative risk for that recipient of remaining on the transplant waiting list compared to that of receiving a DCD organ. (1B)
We suggest that:
- Final consent for transplantation of a DCD organ, where the donor type has a significant impact on expected organ outcomes, should not normally be delegated, particularly given the complexities around the discussion of alternative strategies, like waiting for another organ offer. (2D)
- Units generate consent addenda that adhere to NHSBT/BTS guidance covering risks and benefits specific to transplantation of different organ types that should include information regarding transplantation of organs from DCD donors, where the donor type has a significant impact on expected organ outcomes. (2D)
8.1 Introduction
Valid consent requires that the potential transplant recipient be informed of the risks and benefits of an intervention, namely transplantation using a DCD donor organ. The NHSBT and BTS have jointly produced a Guideline for Consent for Solid Organ Transplantation (1). This provides specific recommendations about the provision of information during patient consent and reflects the challenges that are unique to transplantation such as the diversity of risk versus benefit depending upon organ type, recipient and donor co-morbidity, timeframes for decision making, and limited organ supply. The guideline highlights key areas for consideration to facilitate consent:
- Information to be given prior to joining the transplant waiting list
- Maintaining consent while on the waiting list
- Informing patients about risk
- Patient choice and the donor organ
- Discussions at the time of an organ offer
- Information which the recipient is entitled to know about the donor
- Information which the donor family is entitled to know about the recipient
8.2 Informing the recipient
Informing the recipient is a complex process and individual patients have different requirements for information. The method of delivery must be flexible to reflect this and is best achieved through a multi-disciplinary approach. Specialist nurses/recipient coordinators often take a lead role in providing education and support for potential recipients, but engagement across the multi-disciplinary team is vital. To augment verbal and written communication the use of visual and infographic materials may further aid consent discussions.
8.3 Consent
A two-stage consent process is advocated.
Stage one involves patient registration on the national deceased donor transplant waiting list, accompanied by oral and written information from the multidisciplinary team on the risks and benefits of transplantation. Peer support also provides a valuable opportunity to involve patients who have previously experienced transplantation in the support of those who are embarking upon the process. This complements the approach of healthcare professionals, encourages acceptance of chronic illness, and supports decision-making (1). Best practice recommends that consent and accompanying information are updated annually for recipients who remain on the list (2).
Stage two involves confirmation of consent on admission for transplantation by the transplanting surgeon. From a legal perspective, the surgeon is held accountable for consent and there are a number of issues arising from the judgment in Montgomery v Lanarkshire that apply (3).
The surgeon is under a duty to take reasonable care to ensure that the patient is aware of any material risks involved in any recommended treatment and of any reasonable alternative or variant treatments.
The test of materiality is whether in the circumstances of the particular case a reasonable person in the patient’s position would be likely to attach significance to the risk or the surgeon is or should reasonably be aware that the particular patient would be likely to attach significance to it.
8.4 Specific Considerations for the Recipient of a DCD Organ
The key issue for any potential transplant recipient is to understand the risks and benefits of remaining on the transplant waiting list versus those of accepting the organ on offer (as per a discussion of reasonable alternative or variant treatments).
In the context of DCD, there are organ-specific considerations relating to the type of organ that is required and the characteristics of the individual organ, and recipient considerations such as the likely length of time to wait for an alternative organ and the risk of death while waiting (as per a discussion of material risks).
Where the donor type has a significant impact on expected organ outcomes, the risks involved from DCD organs, and the risks involved in waiting for a non-DCD organ, need to be individualised wherever possible, with data from the transplant unit being supplemented by national data where available. NHSBT offers a useful online risk assessment tool which can help both the patient and transplant clinician quantify many of the risks involved aiming to be an adjunct to decision-making (4). It is pertinent to note that, while the risk of receiving an organ transplant is correctly highlighted in the consent process, the risk of remaining on the transplant waiting list is often significantly underestimated.
It is the responsibility of the treating clinician to obtain consent (although this may be delegated to an appropriately experienced health care professional or team) (1). Final consent for transplantation of a DCD organ, where the donor type has a significant impact on expected organ outcomes, should not normally be delegated, particularly given the complexities around the discussion of alternative strategies, like waiting for another organ offer.
References
- Guidelines for Consent for Solid Organ Transplantation in Adults, NHS Blood and Transplant/British Transplantation Society, March 2011. Available at: https://www.bts.org.uk/
- Hughes J, Wood E, Smith G. Exploring kidney patients’ experiences of receiving individual peer support. Health Expect 2009;12:396-406.
- Montgomery v Lanarkshire Health Board [2015] SC 11 [2015] paragraph 87.
- NHSBT risk communication tool https://www.odt.nhs.uk/transplantation/tools-policies-and-guidance/risk-communication-tools/
9. Kidney
We recommend that:
- The incidence of delayed graft function is 40-50% in recipients of kidneys from DCD donors and this should be discussed with the patient prior to transplantation. (1A)
- Potential recipients should be informed that long-term outcomes for standard criteria donors are equivalent for DCD and DBD kidney transplants. (1A)
- Contraindications to kidney donation do not differ according to deceased donor type. (1B)
- Long-term outcomes of DCD recipients are similar to those of DBD recipients and the allocation system for DCD and DBD donor organs should be similar. Nevertheless, it is recognised that DCD donor kidneys are more susceptible to cold ischaemia than DBD kidneys, and should be implanted <12 hours, where possible. (1B)
- There is no evidence to support the use of alternative immunosuppression strategies in DCD donor kidney transplants beyond the standard of care. (1B)
We suggest that:
- None of the hypothermic machine perfusion perfusate effluent biochemical analysis/perfusion pressure dynamic characteristics, or kidney transplant biopsy scoring systems – alone or in combination – have sufficient predictive value to mandate organ discard. (2A)
- Hypothermic machine perfusion may reduce the incidence of delayed graft function in recipients of DCD donor kidneys when performed from the point of retrieval. (2B)
- The use of kidneys from DCD donors with AKI should be considered in the context of individual recipient factors. (2B)
- The use of kidneys from donors with a withdrawal time more than 3 hours or absent blood pressure for >30 minutes should be restricted to protocols that attempt to resuscitate organ viability. (2C)
- Normothermic regional perfusion may reduce the incidence of delayed graft function and improve kidney function in DCD kidneys. (2B)
- In paediatric recipients, the rate of DGF and PNF is higher in DCD donor kidneys compared to DBD donor kidneys. However, three-year graft survival is comparable. (2B)
9.1 Introduction
Kidney transplantation using DCD organs is fundamentally similar to transplantation using DBD organs, except that the organs will have suffered an additional warm ischaemic insult. The use of normothermic regional perfusion in DCD donor transplantation is associated with a longer asystolic time but the impact of this is nullified by avoiding a period of cold ischaemic time immediately afterwards (see Chapter 7).
Historically, higher rates of primary non-function (PNF) and delayed graft function in DCD donor kidneys were considered to be obstacles to the use of kidneys from this donor type. However, DCD donor kidneys have demonstrated comparable medium-term death-censored graft survival compared to DBD donor kidneys (1). Whilst DGF is associated with inferior graft survival in DBD donor kidneys, DCD donor kidneys appear to be more resilient to the injuries underlying DGF (2).
A landmark UK-based cohort study has suggested that DCD donor kidneys are more susceptible to cold ischaemic injury, but there is no evidence that DCD ECD donor kidneys suffer from more long-term graft dysfunction than equivalent DBD ECD donor kidneys (3).
Deceased donor kidney donation in the UK is currently associated with increasing numbers of both ECD and DCD donors. This necessitates better risk assessment of donor and recipient in order to accurately predict outcomes. As a result, methods of assessing donor and recipient risk are being employed to guide decision-making, namely registry-based risk indices, machine perfusion technologies and histological examination of donor kidneys prior to transplantation.
9.2 Donor Selection
Absolute contraindications
The absolute contraindications for DCD donor kidneys do not differ from DBD donors.
Relative contraindications
With the expansion of the donor pool, the utilisation of kidneys from sub-optimal donors is becoming increasingly common. The use of kidneys that are identified as ‘higher risk’ of graft loss should be weighed against the risks of remaining on the deceased donor kidney transplant waiting list or the realistic probability of receiving a better offer within an acceptable time frame.
Donor and retrieval factors that impact graft outcomes include donor age and cold ischaemic time (1,4). Additional factors such as donor hypertension and cardiovascular disease have also been shown to have an impact on DCD kidney survival, but to a lesser degree (4).
The effect of donor AKI on transplant outcomes has been examined in large registry studies. A large UK-based study (5) showed that rates of primary non-function and delayed graft function (DGF) were not independently associated with DCD versus DBD donation, and reported good outcomes from kidneys with AKI stages 1-2. A more recent US study has shown a higher rate of graft failure in DCD kidneys with AKI stages 1-2 compared to DBD kidneys, but better patient outcomes than remaining on the waiting list (6).
9.3 Donor Warm Ischaemic Times
A recent large cohort study of patients from the UK transplant registry examined 10,000 deceased donor warm ischaemic times, including 3,000 DCD donors (7). This study showed that although DCD donor kidneys were associated with a higher risk of DGF, this was highest when FWIT exceeded 30 minutes, with DBD donors used as a comparator group (OR 5.8 95% CI 2-8-12.1). However, there was no demonstratable difference in PNF or medium-term graft survival.
The use of kidneys from DCD donors with >30 minutes of absent blood pressure may be considered in programmes which are undertaking measures to ‘recondition’ organs via ex situ oxygenated normothermic perfusion, with the possibility of a more predictive assessment of organ quality.
9.4 Organ Retrieval
Retrieval from controlled DCD donors employs a ‘super-rapid’ retrieval technique to minimise warm ischaemic damage, however, there is significant inter-clinician variability in how this is achieved. Inadvertent injury to the kidney is higher in DCD donors relative to DBD donors (8).
9.5 Organ Preservation
Preservation solutions
No definitive data currently suggest any advantage for specific preservation solutions in the context of DCD kidney transplantation (9, 10) despite the wide variation in cost.
9.6 Organ Quality Assessment
After transplantation, kidneys may work immediately, recover after a period of impaired or absent function, or never function at all. Early function is dependent upon the underlying health of the donor as well as the ischaemic time, any damage sustained during the process of death and organ retrieval, as well as operative, immunological, and recipient factors. Because of the availability of dialysis to support initial graft dysfunction, the emphasis in kidney transplantation must be on minimising, and as far as possible eradicating, PNF.
9.6.1 Viability Assessment from Perfusion Parameters and Biomarkers
There has been significant interest in whether information gained during machine perfusion may enable organ viability assessment.
In cold machine perfusion, a European cohort study examined the performance of 336 deceased donor kidneys to determine whether kidney vascular resistance could predict delayed graft function (11). This study showed that renal resistance was an independent predictor of DGF on multivariable analysis. However, analysis of received operator characteristic (ROC) curves showed a low predictive accuracy for DGF (area under the curve 0.58). Existing studies examining early biomarkers of DGF and PNF are limited in quality and have not been found to be highly predictive of post-transplant outcomes (12).
In ex situ warm machine perfusion, kidneys are thought to be put in a functional state, allowing urine output, and macroscopic appearance to be examined, in addition to kidney blood flow. In a study of 74 human kidneys, critical thresholds associated with superior graft function were determined (13). Performance of a kidney over 60 minutes of warm perfusion enables a quality assessment score (Table 9.1), albeit subjective in part.
Kidneys scoring 1 are considered the highest quality, and kidneys scoring 1-3 are considered to be safe for transplantation. Kidneys scoring 4-5 were considered untransplantable due to the high likelihood of primary non-function. The use of this score was further validated in a second UK multi-centre study (14).
Early work examining biomarkers in urine produced during warm and cold machine perfusion has also shown potential for organ viability assessment (15).
Table 9.1 Scoring criteria of kidneys on ex situ warm machine perfusion (13).
| Perfusion parameter | Score | |
| Macroscopic appearance | ||
| Excellent perfusion: global pink appearance | 1 | |
| Moderate perfusion: patchy appearance | 2 | |
| Poor perfusion: globally mottled, purple or black appearance | 3 | |
| Renal blood flow (adjusted to organ weight) | ||
| ≥ 50 mL/min/100g | 0 | |
| < 50 mL/min/100g | 1 | |
| Urine output | ||
| ≥ 43 mL/min/hour | 0 | |
| <43 mL/min/hour | 1 | |
9.6.2 Viability Assessment From Biopsy Parameters
Work on the assessment of organ quality from histological parameters is mainly derived from the examination of ECD, rather than specifically in relation to DCD, organs. There is conflicting evidence on whether the routine use of pre-implantation kidney biopsies improves graft outcomes. The effect of provision of a national donor kidney histology service on the organ utilisation of kidneys offered from deceased donors aged over 60 years is currently being investigated (Pre-Implantation Trial of Histopathology In renal Allografts – PITHIA; ISRCTN11708741) (16).
Composite scores combining donor histology with other donor and recipient characteristics may provide the best predictive value. An international cohort study examining eight functional, histological and immunological prognostic factors have been combined to produce a graft survival prediction score with good predictive accuracy (C-statistic 0.81) (17).
There are currently no histological markers that predict PNF as a result of excess warm ischaemia or irreversible ischaemia-reperfusion.
9.6.3 Clinical Donor Risk Scores
A range of increasingly complex scoring systems have been developed in an attempt to predict outcomes in relation to pre-existing donor factors. These are not specific for use in the context of DCD kidney donation(18). No scoring system, either alone or in combination with pump parameters or histological scoring, has yet been shown to accurately define which organs should be discarded due to an excessive risk of PNF or seriously impaired long-term graft function (1).
9.7 Ex situ Machine Perfusion
A recent Cochrane review and meta-analysis has shown that cold machine perfusion is protective against delayed graft function in DCD donor kidneys (RR 0.75, 95% CI 0.64-0.87) (19). This work suggests that seven perfusions are needed to achieve immediate graft function in one DCD donor kidney, compared to static cold storage alone. There is no demonstrable advantage of oxygenated hypothermic machine perfusion compared to non-oxygenated in expanded criteria donors (20).
A multicentre open-label randomised control trial examined the use of ex situ warm machine perfusion prior to DCD donor kidney transplantation, compared to static cold storage alone (21). Kidneys randomised to warm machine perfusion were perfused for one hour with an oxygenated red blood cell-based solution at 36.0°C. There was no demonstrable reduction in delayed graft function between kidneys undergoing warm machine perfusion compared to static cold storage alone.
9.8 Recipient Selection
In the UK, current data suggest that recipients of DCD kidneys have similar longer-term outcomes to recipients of DBD kidneys (1). UK registry data show that the incidence of DGF in DCD recipients ranges from 39-50% compared with 25% in DBD recipients (1, 22).
9.8.1 Factors that May Influence Outcome
NHSBT data show that increasing donor and recipient age and a cold ischaemic time of >12 hours are associated with a worse outcome (1). ECD kidneys are associated with inferior graft survival compared to SCD kidneys, irrespective of DBD / DCD status (23).
9.9 Immunosuppression
Use and choice of induction therapy
Induction therapy with either IL2-receptor blockade or lymphocyte depletion has previously been shown to reduce DGF in retrospective studies of DCD kidney transplants (24, 25), and has been recommended in earlier guidelines. No single agent has been shown to be superior in the setting of DCD kidneys.
More recently induction therapy with basiliximab is recommended in NICE guidance (Immunosuppressive Therapy for Kidney Transplants in Adults, 2017) and is now considered to be standard of care for the majority of transplants in UK transplant centres, regardless of donor type.
Choice of calcineurin inhibitor
The studies comparing immunosuppression regimens in DCD renal transplantation are largely retrospective, and from an era when DCD outcomes were inferior to now. The theoretical advantages of minimising exposure to calcineurin inhibitors (CNIs) in the early post-operative period have not been convincingly demonstrated in practice to date.
Early sirolimus use is associated with increased adverse events including prolonging delayed graft function and acute rejection (26) and is not recommended.
Belatacept has not been studied in large numbers of DCD kidney transplants. Further prospective studies in the current era (with monoclonal antibody induction therapy and tacrolimus as the standard of care) are required to determine the optimum immunosuppressive strategy; currently, there is no evidence to support the use of alternative regimens in DCD transplants.
Overall graft outcome after transplantation is primarily determined by the quality of the donor rather than the mode of donation (Figures 9.1 and 9.2). Thus, graft outcome is more closely related to whether the donor has additional risk factors (e.g., is ECD versus SCD) than whether the mode of retrieval is DCD versus DBD (23).
9.10 Outcomes
Figure 9.1 Graft survival following kidney transplantation from different donor types (23).
As well as graft survival, longer-term graft function is also similar. GFR is initially poorer because of the high incidence of DGF in DCD, but is equivalent after 3 months.
Figure 9.2 Graft survival following kidney transplantation from different donor types (1).
Despite the increased incidence of DGF in recipients of DCD donor kidneys, there does not appear to be a graft or patient survival disadvantage in the majority of DCD donor kidney recipients relative to DBD donor kidney recipients (1, 2). Considering DGF duration in DCD donor kidney transplantation reveals that DGF lasting <14 days is not associated with inferior graft or patient survival (22). In DCD donor kidney transplantation, recipients with DGF lasting >14 days are at a significantly higher risk of graft and patient loss. In a UK registry study, 5,954 first kidney-only transplants were undertaken from DCD donors and NRP was used prior to 210 kidney transplants (4%). In risk-adjusted analyses, NRP kidneys had a 35% lower chance of developing delayed graft function than non-NRP kidneys (odds ratio, 0.65; 95% CI, 0.47-0.90)‚ and the expected 12-month estimated glomerular filtration rate was 6.3 mL/min/1.73m2 better if abdominal NRP was used (p< 0.0001) (27).
9.11 Paediatrics
The use of DCD donor kidneys for paediatric recipients is relatively uncommon, making up <2% of paediatric kidney transplants, primarily due to concerns about PNF and DGF. In paediatric recipients, the rate of PNF is 5% and the rate of DGF is 25% (28). However, following cautious DCD donor selection, there are comparable three-year survival rates to matched DBD donor kidney recipients.
References
- Summers DM, Johnson RJ, Allen J, et al. Analysis of factors that affect outcome after transplantation of kidneys donated after cardiac death in the UK: a cohort study. Lancet 2010;376(9749):1303-11.
- de Kok MJ, McGuinness D, Shiels PG, et al. The neglectable impact of delayed graft function on long-term graft survival in kidneys donated after circulatory death associates with superior organ resilience. Ann Surg 2019;270(5):877-83.
- Summers DM, Johnson RJ, Hudson A, et al. Effect of donor age and cold storage time on outcome in recipients of kidneys donated after circulatory death in the UK: a cohort study. Lancet 2013;381(9868):727-34.
- Reid AW, Harper S, Jackson CH, et al. Expansion of the kidney donor pool by using cardiac death donors with prolonged time to cardiorespiratory arrest. Am J Transplant 2011;11(5):995-1005.
- Liu C, Hall IE, Mansour S, et al. Association of deceased donor acute kidney injury with recipient graft survival. JAMA Netw Open 2020;3(1):e1918634.
- Lia D, Singer P, Nair V, et al. DCD renal transplantation from donors with acute kidney injury. Transplantation 2021;105(4):886-90.
- Kostakis ID, Kassimatis T, Flach C, et al. Hypoperfusion warm ischaemia time in renal transplants from donors after circulatory death. Nephrol Dial Transplant 2020;35(9):1628-34.
- Ausania F, Drage M, Manas D, et al. A registry analysis of damage to the deceased donor pancreas during procurement. Am J Transplant 2015;15(11):2955-62.
- Timsit MO, Tullius SG. Hypothermic kidney preservation: a remembrance of the past in the future? Curr Opin Organ Transplant 2011;16(2):162-168.
- O’Callaghan JM, Knight SR, Morgan RD, et al. A national registry analysis of kidney allografts preserved with Marshall’s solution in the United Kingdom. Transplantation 2016;100(11):2447-52.
- Jochmans I, Moers C, Smits JM, et al. The prognostic value of renal resistance during hypothermic machine perfusion of deceased donor kidneys. Am J Transplant 2011;11(10):2214-20.
- Guzzi F, Knight SR, Ploeg RJ, et al. A systematic review to identify whether perfusate biomarkers produced during hypothermic machine perfusion can predict graft outcomes in kidney transplantation. Transpl Int 2020;33(6):590-602.
- Hosgood SA, Barlow AD, Hunter JP, et al. Ex vivo normothermic perfusion for quality assessment of marginal donor kidney transplants. Br J Surg 2015;102(11):1433-40.
- Hosgood SA, Thompson E, Moore T, et al. Normothermic machine perfusion for the assessment and transplantation of declined human kidneys from donation after circulatory death donors. Br J Surg 2018;105(4):388-94.
- Hosgood SA, Hunter JP, Nicholson ML. Early urinary biomarkers of warm and cold ischemic injury in an experimental kidney model. J Surg Res 2012;174(2):e85-90.
- Ayorinde JO, Summers DM, Pankhurst L, et al. PreImplantation Trial of Histopathology In renal Allografts (PITHIA): a stepped-wedge cluster randomised controlled trial protocol. BMJ Open 2019;9(1):e026166.
- Loupy A, Aubert O, Orandi BJ, et al. Prediction system for risk of allograft loss in patients receiving kidney transplants: international derivation and validation study. BMJ 2019;366:l4923.
- Watson CJ, Johnson RJ, Birch R, et al. A simplified donor risk index for predicting outcome after deceased donor kidney transplantation. Transplantation 2012;93(3):314-8.
- Tingle SJ, Figueiredo RS, Moir JA, et al. Machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. The Cochrane Database Syst Rev 2019;3:Cd011671.
- Husen P, Boffa C, Jochmans I, et al. Oxygenated end-hypothermic machine perfusion in expanded criteria donor kidney transplant: a randomized clinical trial. JAMA Surg 2021;156(6):517-25.
- Hosgood, S.A., Callaghan, C.J., Wilson, C.H. et al. Normothermic machine perfusion versus static cold storage in donation after circulatory death kidney transplantation: a randomized controlled trial. Nat Med 2023; 29, 1511–1519.
- Phillips BL, Ibrahim M, Greenhall GHB, et al. Effect of delayed graft function on longer-term outcomes after kidney transplantation from donation after circulatory death donors in the United Kingdom: A national cohort study. Am J Transplant 2021;21(10):3346-55.
- Montero N, Toapanta N, Pallarès N, et al. Deciphering transplant outcomes of expanded kidney allografts donated after controlled circulatory death in the current transplant era. A call for caution. Transpl Int 2021;34(12):2494-506.
- Teraoka S, Nomoto K, Kikuchi K, et al. Outcomes of kidney transplants from non-heart-beating deceased donors as reported to the Japan Organ Transplant Network from April 1995-December 2003: a multi-center report. Clin Transpl 2004:91-102.
- Sánchez-Fructuoso AI, Marques M, et al. Use of different immunosuppressive strategies in recipients of kidneys from nonheart-beating donors. Transpl Int 2005;18(5):596-603.
- Asher J, Vasdev N, Wyrley-Birch H, et al. A prospective randomised paired trial of sirolimus versus tacrolimus as primary immunosuppression following non-heart beating donor kidney transplantation. Curr Urol 2014;7(4):174-80.
- Oniscu GC, Mehew J, Butler AJ, et al. Improved organ utilization and better transplant outcomes with in situ normothermic regional perfusion in controlled donation after circulatory death. Transplantation 2023 Feb 1;107(2):438-48.
- Marlais M, Pankhurst L, Hudson A, et al. UK national registry study of kidney donation after circulatory death for pediatric recipients. Transplantation 2017;101(6):1177-81.
10. Liver
We recommend that:
- All centres should be prepared to use livers from DCD donors for transplantation. (1B)
- The outcome of transplanting DCD livers recovered without normothermic regional perfusion is improved with short CIT, and that CIT should be kept under 8 hours. (1B)
- Ex situ preservation time can be extended beyond 8 hours when normothermic regional perfusion has been used. (1B)
- Ex situ preservation time can be extended beyond 8 hours when ex situ machine perfusion is used, but the impact on ischaemic cholangiopathy is unknown. (1B)
- If the donor functional warm ischaemia time exceeds 30 minutes and organs are not being recovered using in situ normothermic regional perfusion or ex situ hypothermic machine perfusion, there is an increased risk for graft loss, however further donor and recipient characteristics should be taken into account before considering rejecting the graft in borderline cases. (1B)
- The use of in situ normothermic regional perfusion and ex situ hypothermic machine perfusion can reduce the incidence of symptomatic ischaemic cholangiopathy when compared to static cold storage. (1A)
- Future studies comparing the effects of different machine perfusion technologies on the biliary tree in DCD donors as well as studies to find adequate assessment parameters of the viability of the biliary tree are necessary. (1A)
- The use of normothermic regional perfusion is an effective way to increase the number of viable livers recovered. (1B)
- Future studies evaluating the possible mechanisms protecting against ischaemic cholangiopathy in DCD donors are needed. (1B)
- Excellent short- and medium-term outcomes can be achieved in paediatric DCD liver transplantation with highly selected and careful donor and recipient selection. (1B)
- Use of paediatric or young adult DCD grafts is an effective approach to expand the donor pool and remains an underutilised resource for children in need of liver transplantation. (1B)
- An international registry of paediatric DCD liver transplant recipients is needed to determine whether there is a significant difference in outcomes from DBD transplantation. (1B)
- A national differential analysis of outcomes in paediatric DCD liver transplantation is required, depending on whether a paediatric or an adult DCD is used. (1B)
We suggest that:
- DCD donors may be used without an age limit if other surrogates of donor organ quality are favourable. (2B)
- High donor BMI is a risk factor for graft loss both in DCD and DBD donation, therefore a higher BMI alone should not be a contraindication for accepting a DCD graft if other factors are favourable. (2B)
- Serial blood gases during the withdrawal phase should be used as an additional tool to determine the onset of anaerobic respiration by providing lactate measurements. (2D)
- The total preservation time could be extended beyond 8 hours if any of the machine organ preservation techniques are utilised, but there is no recommendation of a safe upper limit of preservation based on current evidence and this should be at the discretion of the implanting surgeon. (2D)
- Potential recipients of DCD liver grafts which have not been subject to in situ or ex situ perfusion should be informed of the potential risk of both early and late graft loss. (2C)
- The emphasis should be on minimising donor hepatectomy time; in ideal scenarios, hepatectomy time should not be longer than 30 minutes. A longer hepatectomy time is associated with graft failure in non-NRP DCD donors. (2C)
- Dual aortic and portal perfusion during DCD liver retrieval and flushing of the bile duct should be standard. (2C)
- Time between knife-to-skin and liver placement into the ice box should ideally be less than one hour; this is a target that all NORS teams should be encouraged to achieve. (2B)
- DCD livers can be used in children as whole, reduced or split grafts, if they are of excellent quality, the FWIT is <30 minutes and the CIT <8 hours. (2B)
- Paediatric DCD livers recovered without normothermic regional perfusion are less likely to present with ischaemic cholangiopathy as they may be more resilient to ischaemia reperfusion injury and have higher regenerative capacity. (2B)
- Machine perfusion in paediatric liver transplantation can play a role in halting the effects of the CIT, improving the liver quality in whole grafts for older children or young adults, and facilitating splitting and utilisation of both lobes. (2C)
10.1 Introduction
The burden of chronic liver disease continues to grow worldwide and the UK is no exception. Consequently, the indications for liver transplantation continue to expand with increasing numbers of patients listed, though there is a continuing shortage of donor livers. The liver transplant waiting list mortality in the UK is currently 8-10% per annum (1).
The use of livers from DCD donors has increased considerably in recent years and has come to represent almost 20% of liver transplantations performed in the UK (1). The majority of these grafts continue to undergo super rapid recovery, however the use of preservation machines, both in situ and ex situ, have expanded further the utilisation of these grafts.
Historically, the long-term outcome for liver transplantation from DCD donors has been inferior to livers from DBD donors. However, comparable outcomes for DBD and DCD livers after transplantation can be achieved (2). It is also becoming apparent that the outcome of DCD livers depends on a number of donor and recipient factors, as well as technical factors in relation to the organ retrieval and implantation.
10.2 Donor Selection
Donor age has played an important role in risk stratification, and in the early era, donors more than 50-60 years old were considered high risk (4,5). Recent experience and published literature suggest that good outcomes with older DCD can be achieved (6). Therefore, the influence of donor age (namely >60 years) on the results of liver transplantation using a DCD graft is debatable.
Adoption of a maximum age limit and expanding the donor pool are dependent on many factors, including the centre policy and the burden on the transplant waitlist. Centres that have conservative approaches should be encouraged to expand their donor selection criteria because the overall benefit will then lean towards reducing the national wait list (3).
A previous study has reported comparable outcomes including ischaemic cholangiopathy with DCD grafts from donors older than 60 years old (4). A further single centre series found no difference in complications when comparing livers transplanted from donors less than 70 years old compared with donors >70 years old(6). On the other hand, a retrospective cohort study performed on the Eurotransplant database highlighted a linear association between donor age and graft failure with the increased risk of graft failure associated with prolonged CIT (7). We would suggest the use of DCD donors without age limit if the rest of the donor demographics and other variables (e.g., short ischaemic times) are favourable.
Donors with high BMI are considered to be at risk of graft loss in DCD transplantation as well as DBD. A higher BMI is likely to be a surrogate for steatosis in liver grafts. Previous experience has shown a negative impact of donor BMI >25 kg/m2 at any donor age on graft and patient survival (4). However, more recent studies have demonstrated good outcomes with DCD grafts from donors with BMI >35 kg/m2 (8). We would suggest that donor BMI alone should not be a decision-making factor in DCD liver transplantation.
10.3 Donor Warm Ischaemic Times
The withdrawal time is defined as the time from donor withdrawal of treatment to initiation of cold perfusion or the start of normothermic regional perfusion (see Chapter 3, section 3.2). The functional warm ischaemia time (FWIT) starts when the systolic blood pressure has a sustained (i.e., at least 2 minutes) fall below 50 mmHg and extends up to the onset of cold in situ perfusion or normothermic regional perfusion. There are several published definitions in the literature as well as several analyses regarding the relationship between post-transplant graft loss and ischaemic cholangiopathy. Absolute cut-off values to define the onset of FWIT are less clear in the published literature as different countries have different thresholds.
The UNOS registry identifies FWIT as the period of time from when donor SBP drops below 80 mmHg and/or donor SpO2 drops below 80% (9). A period of time longer than 30 minutes has been considered an increased risk for graft loss.
On the other hand, publications from the SRTR (Scientific Registry of Transplant Recipients) data have identified that livers with withdrawal to perfusion time (termed by them as the donor warm ischaemic time, DWIT) of between 30-40 minutes showed similar graft survival compared to livers from DCD donors with <30 minutes. It would be reasonable to consider the use of DCD livers from donors with withdrawal to perfusion times slightly longer than 30 minutes in selected recipients (10), in the absence of NRP. FWIT with SpO2 <60% was found on univariate analysis among patients who developed a post-transplant complication. When controlling for the effect of CIT and donor age, FWIT was not found to be a significant predictor of the development of post-transplant complications (11). The hypothesis is that ischaemic hepatic injury is more closely related to the onset of hypoxia rather than hypotension. Hypoxia would be more detrimental to DCD donor livers than hypotension during the agonal phase; and a drop in SpO2 to <80% is much more relevant to assess the potential severity of hepatic ischaemia-reperfusion injury (IRI). The rationale behind this is that the lack of oxygenated metabolism leads to energy depletion; the anaerobic metabolism is insufficient to meet energy demand, culminating in cellular oedema and death. However, the accuracy of pulse oximetry is limited in the setting of severe hypoxia or hypotension, which is an issue with the evidence in this area (see Chapter 3, section 3.2). We therefore suggest that serial arterial blood gases during the withdrawal phase should be used as an additional tool to determine the onset of anaerobic respiration by providing lactate measurements above normal range or rising from baseline. One of the first analyses on the field of warm ischaemia time and use of DCD grafts comes from Ho et al in 2008 (12). This study (among the few multicentre studies) examined DCD donor post-extubation hypotension and subsequent liver and kidney graft outcomes in terms of graft survival. This paper was the first one to report that the time period from SBP less than 50 mmHg to aortic flush was the best predictive test for graft survival. Another multicentre study published by Coffey et al has measured FWIT as SBP less than 50 mmHg, however, no association with graft survival was analysed (11).
The previous British Transplantation Society DCD guidelines defined the start of FWIT to be when SpO2 drops below 80% or SBP <50 mmHg and it recommended not to use liver grafts for transplantation if this time exceeded 30 minutes (12). It was seen in previous studies that the duration of SBP <50mmHg is directly correlated with increased rates of ischaemic cholangiopathy, graft loss or death and there is no new solid evidence to suggest that these thresholds should be changed when traditional rapid recovery is employed(13).
We suggest that the onset of FWIT is defined as the time period between the systolic blood pressure drop <50mmHg following the withdrawal of life support, until aortic cold flush is instituted or NRP is commenced.
10.4 Donor Hepatectomy Time, and Time Between Donor Hepatectomy and Into the Ice Box
Maintaining the organ temperature is key in organ preservation and has been linked to PNF. Once cross-clamping is performed, arterial and portal perfusion is started in addition to ice slush in the abdominal cavity. Evidence suggests that organ core temperature is lowered down to 16°C within approximately 11 minutes after initiation of organ cold flush (14). During the next phase, the liver is explanted and by the end of this phase, liver temperature descends to 10°C. The final desired core temperature of 4°C is achieved and maintained only after the liver is packed and inserted in the transport box. Until the liver has cooled down it will continue to experience a higher metabolic rate in an ischaemic environment. It takes a short period of time to cool livers down to 10°C but after flushing is stopped, temperature depression is markedly decreased. An experimental study has observed that static storage at +1°C improved liver function compared with +4°C or -0.5°C (15).
In a study performed by Farid et al, the median donor hepatectomy time (DHT – time between start of cold perfusion to liver on the back-table) was 35 minutes and in the multivariate analysis, the factors associated with graft survival were hepatectomy time longer than 60 minutes, donor older than 45 years, CIT longer than 8 hours and recipient with previous abdominal surgery (16).
Goussous et al have described in a comparative retrospective review (historic group before 2014 and modern group after 2014) that when DHT was dichotomized at <22 minutes versus >22 minutes, a longer DHT was associated to development of ischaemic cholangiopathy (IC) (17).
Jochmans et al found that DCD liver grafts are more susceptible to poor outcomes compared to DBD when DHT is longer. If DHT is prolonged, there will be insufficient cooling in the abdominal cavity leading to rewarming of the graft, with the initiation of ischaemic injury to the donor liver, impacting the graft survival (18).
Evidence is still less clear at which point exactly the DHT starts to have a detrimental effect. Gilbo et alsuggested that there is a linear relationship between DHT and IC and there is a 19% increment in the rate of IC for every 10 minutes increase in DHT (this effect is similar to a one-hour increase in CIT) (19). Other studies have reported that DHT is a significant independent risk factor for IC after DCD liver transplantation (20).
A key period is also the time from aortic/portal flush in situ until the liver is placed into the transport ice box and in the previous study that time was a median of a further 33 minutes (19). Although the fluid in the bowl is cold this is not enough to guarantee the rewarming of the graft doesn’t occur causing a deleterious effect on the bile duct viability. Therefore, we would suggest aiming for no more than one hour from knife-to-skin to liver placement into the ice box time as an achievable target that all NORS teams should be encouraged to achieve. This requires good coordination, communication and teamwork between surgeons and other members of the scrub team.
10.5 Use of Ex situ Machine Perfusion
Ex situ machine perfusion is a preservation method occurring outside the donor’s body, developed to protect organs from the detrimental effects of ischaemia-reperfusion injury (IRI), enable assessment of function, and facilitate the repair/regeneration of DCD grafts in order to expand the donor pool and improve graft function after liver transplantation. Ex situ machine perfusion may be at normal body temperature (normothermic) using a blood-based perfusate, this is termed normothermic machine perfusion (NMP). Ex situ machine perfusion may also be hypothermic, termed hypothermic machine perfusion (HMP). The current evidence favours oxygenating the perfusate, a technique called hypothermic oxygenated machine perfusion (HOPE), running at a temperature of 4 to 10°C. NMP offers the advantage to assess graft viability under more physiologic conditions (21).
To date, there are no clinical trials directly comparing SCS to NMP specifically for DCD livers. The first randomised controlled trial comparing SCS and NMP was the COPE study, which included DBD and DCD liver grafts. The rate of non-anastomotic strictures in the NMP DCD group (11.1%) was non-significantly lower than in SCS (26.3%) despite longer functional warm ischaemia time, although the study was not powered sufficiently to show any statistically significant difference in this outcome measure (22).
A retrospective study analysing the rate of discarded livers concluded that NMP reduces the discard rate of procured livers despite its use in donors traditionally considered of more marginal quality. In that study the NMP group had a 3.5% discard rate versus 13.3% in the SCS group and this was despite NMP donors being older and more frequently DCD (18% vs 7%, p<0.001). The second most common reason for liver discard in the SCS group was DCD warm ischemic time (11%) (23).
In one multicentre, randomised controlled trial, patients received transplantation of a liver obtained from a DCD donor either after dual hypothermic oxygenated machine perfusion (machine perfusion group) or after conventional static cold storage alone (control group). The authors used a dual perfusion approach (both portal vein and hepatic artery) and the argument for this is that the bile duct is dependent on the delivery of oxygen through the hepatic artery. However, previous studies using only HOPE (without hepatic artery perfusion) stated that perfusion through portal vein of high concentration of oxygen would be enough to supply the entire graft. They have described a lower risk of symptomatic non-anastomotic biliary stricture after using hypothermic oxygenated machine perfusion of 6% compared to 18%, and a four-fold decrease in the number of treatments for this complication. Interestingly, the rate of asymptomatic biliary abnormalities was high (~60%) for both groups (24,25).
Protective effects of HOPE or dual perfusion HOPE (DHOPE) were investigated in a retrospective study in extended criteria donor (ECD) DBD and overextended WIT DCD grafts. This retrospective case series included 50 grafts subjected to end-ischaemic HOPE or DHOPE. All the DCD grafts were subjected to normothermic regional perfusion before organ procurement. Cold preservation time greater than 9 hours was associated with early allograft dysfunction (EAD) indicating the need to reduce cold preservation time in the setting of DHOPE (26).
A retrospective multicentre comparative outcomes study (one Swiss and six French centres) compared grafts recovered from NRP with grafts preserved in hypothermic machine perfusion. Both techniques achieved similar one year post-transplant recipient and graft survival rates exceeding 85% and comparable to the benchmark values observed in standard DBD liver transplantation (27). An Italian retrospective study comparing DCD livers recovered after NRP+DHOPE compared with conventional static cold storage subjected to long warm ischaemia time, as per the Italian policies, showed a lower incidence of acute kidney injury in the NRP-DHOPE group, but no statistical differences regarding IC (28).
We consider that before engaging in further RCT comparing different types of MP or SCS, surrogate biomarkers as endpoints need to be better defined, mainly in the DCD field. The concept of EAD defined following a cohort of DBD grafts cannot be applied and expect the same utility in a DCD cohort (29). Also, the use of AST/ALT as an endpoint in these types of studies should not be advised as they reflect hepatocyte damage and they are of very limited value in predicting biliary complications. Bile duct injury has emerged as the most relevant factor determining the performance of an organ in the DCD and machine perfusion era.
We recommend that machine perfusion technologies, regardless of the type, have allowed safe transplantation of DCD liver grafts. However, evidence is less clear on which technique is superior and preventive of ischaemic cholangiopathy as there is a lack of properly randomised studies. So, the use of machine perfusion technology should be based on individual centre preference and experience.
10.6 Organ Retrieval Specifics
10.6.1 Preservation Solution
In order to minimise the detrimental effects of warm ischaemia, the abdominal organs are required to be immediately flushed with an ice-cold preservation solution as soon as possible after death has been declared. As a result of the donor warm ischaemia, ATP levels are progressively depleted.
University of Wisconsin (UW) solution has been used for the majority of DCD donor procedures in the UK, in contrast to data from the European Liver Transplant Registry where cold organ flush was performed using mainly HTK. A retrospective and comparative study related to DCD liver retrieval concluded that HTK compared with the use of UW has a higher rate of biliary complications (30). Data suggest that the use of HTK may be associated with higher liver graft loss in cases where CIT is estimated to be more than 8 hours (31,32).
10.6.2 Aortic-only Versus Dual Perfusion
Regarding aortic versus dual perfusion, the latter should in theory achieve more comprehensive liver perfusion and cooling at a faster rate, however, the final liver temperature appears to be very similar to that achieved via aortic flush alone. Some authors have recommended dual perfusion during DCD liver retrieval, and this is recommended in NHSBT guidance. Although the evidence supporting this practice is relatively limited (33,34), we suggest that dual perfusion during DCD liver retrieval should be the standard.
10.7 Immunosuppression
The utilisation of DCD grafts is associated with increased ischaemia-reperfusion injury predisposing to both acute and chronic renal injury. There is a theory that induction therapy with antibodies may provide an umbrella of protection allowing the delayed introduction of calcineurin inhibitors. However, there is currently no evidence indicating that there should be a difference in the immunosuppression protocol for DCD when compared to DBD liver transplantation.
10.8 Outcomes
We have observed an improvement in graft survival in DCD recipients over the last decade, mainly in the first year of transplantation (1). An improvement in surgical and endoscopic techniques, reduction in ischaemic times and better recipient selection have considerably contributed to this improvement. Trends for DCD outcomes according to two different eras (2008-2011 and 2012-2016) have been reported. In the second era, there were no differences in mortality rates between those receiving a DCD versus a DBD graft (2). One of the major improvements from a procurement and organ preservation point of view has been the use of normothermic regional perfusion combined and/or ex situ machine perfusion. The use of NRP has improved not only organ utilisation but also survival outcomes. From 1470 DCD first liver transplants, 94 (6%) were recovered in NRP. In the NRP group, the event death or graft loss within 12 months was 7% versus 13% in the non-NRP group (35).
10.9 Paediatric Liver Transplantation
The burden of the paediatric liver transplant waitlist does not exceed 50 patients and waitlist mortality is less than 5% per annum. For reasons of early inferior outcomes, the use of DCD liver grafts in the paediatric population should be done only in highly selective situations.
Historically, the use of DCD grafts has been associated with higher risks of PNF, IC, acute kidney injury, vascular thrombosis, and lower graft survival (36,37), hence, the reluctance to use DCD donors for liver transplantation in the paediatric population. With better donor selection and strict acceptance criteria, similar outcomes to those from DBD donors have been achieved with adequate donor and recipient selection (38).
10.9.1 Donor Selection
Two centres in the UK have published outcomes of DCD liver transplantation in children, and both suggest highly selective donor criteria (39,40). Both centres concluded that donor selection is paramount to achieving good outcomes. The recommended criteria considered for appropriate donor selection in children in the UK are:
– Donor age <40-45 years-old
– ITU stay <5 days
– Normal liver function tests
– FWIT <30 minutes
– Normal liver appearance and perfusion after recovery
The graft type could be a full graft if the donor size matched the intended recipient, or a cut-down (reduced size liver). Splitting an organ to two transplantable lobes is not recommended as there is no data to support this approach.
10.9.2 Recipient Selection
Despite the good outcomes reported by different centres, the decision to use DCD livers in critically ill children should be taken carefully, analysing the risk-benefit case-by-case, and when a good quality DBD liver or a suitable living donor is not available in a timely manner.
The main controversies when utilising DCD livers in children are the difficulty to find a size-matched graft in case of requiring an emergency re-transplantation for PNF or hepatic artery thrombosis (HAT), and the unknown long-term outcomes of these grafts in patients likely to have a longer life expectancy when compared to the adult population.
10.9.3 Outcomes
Despite the limited experience around the world in paediatric DCD liver transplantation, single-centre series reported by large volume transplant units have published patient and graft survival rates of 100% at three-years follow-up, with no increased incidence of PNF, vascular complications or biliary complications (39,40).
A multi-centre retrospective study in the Netherlands reported the outcomes of 20 paediatric DCD donors used in either children or adults. They reported a graft survival rate of 65% at one year in the DCD group, compared to 82% at one year in the DBD group, with most cases of graft failure occurring in the first three months. When excluding those donors with FWIT >30 minutes, one-year graft survival rate in DCD LT was 81%, like the DBD group. All cases with FWIT >30 minutes lost the graft because of PNF or HAT.
In the Dutch study, the incidence of IC in paediatric DCD grafts was relatively low and similar to the incidence in paediatric DBD grafts (5% versus 4% respectively) and lower than the incidence of IC in adult DCD LT. These findings suggest that the regenerative capacity is better preserved in younger donors and that the biliary epithelium in children is more resilient to ischaemia and reperfusion injury (41).
Long-term complications were observed in two patients who had biochemical evidence of persisting cholestasis, both of whom had histological features of chronic rejection. No difference was observed in the prevalence of post-transplant cholestasis in paediatric recipients of DCD livers depending upon the type of biliary enteric anastomosis. In the presence of a normal recipient bile duct, choledocho-choledochostomy is the preferred method. However, due to the large number of children with biliary atresia, or the small size of the bile duct, Roux-en-Y hepatico-jejunostomy is commonly required.
10.9.4 Machine Perfusion in Paediatric Liver Transplantation
The first successful paediatric DCD LT after hypothermic machine perfusion has been reported by a team in the Netherlands. A whole DCD liver from a 13-year-old donor underwent a dual combined arterial and portal hypothermic oxygenated ex situ for two hours before implantation in a 16-year-old patient with progressive familial cholestasis. After one year of follow-up, the liver function and a routine liver biopsy were completely normal (42).
Different authors have shown the feasibility of splitting a DBD liver during HOPE, with the possibility of removing the left lateral segment from the circuit first, allowing the right lobe to stay perfused until it is utilisedor put in the ice box to be transported to another centre. All grafts were successfully transplanted in adult and paediatric recipients, but further evidence in DCD livers and careful implementation is required (43,44).
The feasibility of splitting livers during ex situ normothermic machine perfusion has also been demonstrated(45). The group from Birmingham published the first study demonstrating that splitting a DCD liver during NMP is feasible and viability is maintained when measuring good viability criteria separately in both hepatic arteries(46).
In summary, current experience shows that good outcomes can be achieved in paediatric liver transplantation from DCD donors, with lower risk of PNF and ischaemic cholangiopathy when donors are selected carefully. Although data are limited, it is also suggested that the outcomes when using DCD grafts from paediatric donors are better than adult DCD donors. Satisfactory outcomes of whole, reduced or split grafts from selected young DCD donors have been reported in small children if the CIT is kept below 8 hours and FWIT <30 minutes. Further experience is needed before recommending a wider practice of this approach.
References
- Annual report on liver transplantation. Report for 2020/2021. NHSBT https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/24593/nhsbt-liver-transplant-report-2021-final.pdf
- Wallace D, Cowling TE, Suddle A, et al. National time trends in mortality and graft survival following liver transplantation from circulatory death or brainstem death donors. Br J Surg 2021 Dec 17;109(1):79-88.
- Cascalaes Campos P, Ferreras D, Alconchel F, et al. Controlled donation after circulatory death up to 80 years for liver transplantation: pushing the limit again. Am J Transplant 2020; 20(1):204-12
- Schlegel A, Scalera I, Perera MTPR, et al. Impact of donor age in donation after circulatory death liver transplantation: Is the cutoff ‘’60’’ still of relevance? Liver Transplant 2018;24:352-62.
- Croome KP, Mathur AK, Lee DD, et al. Outcomes of donation after circulatory death liver grafts from donors 50 years or older: a multicentre analysis. Transplantation 2018;102:1108-14.
- Giorgakis E, Khorsandi S, Mathur A, et al. Comparable graft survival is achievable with the usage of donation after circulatory death liver grafts from donors at or above 70 years of age: a long term UK national analysis. Am J Transplant 2021;21:2200-10.
- Pratschke S, Bender A, Boesch F, et al. Association between donor age and risk of graft failure after liver transplantation: an analysis of the Eurotransplant database. Transpl Int 2019;32:270-9.
- Mihaylov P, Mangus R, Esker B, et al. Expanding the donor pool with the use of extended criteria donation after circulatory death livers. Liver Transplant 25;8: 1198-208.
- Mateo R, Cho Y, Singh G, et al. Risk factors for graft survival after liver transplantation from donation after cardiac death donors: an analysis of OPTN/UNOS data. Am J Transplant 2009; 9:773-81.
- Paterno F, Guarrera JV, Wima K, et al. Clinical implications of donor warm and cold ischemia time in donor after circulatory death liver transplantation. Liver Transplant 25;9:1342-52.
- Coffey JC, Wanis KN, Monbaliu D, et al. The influence of functional warm ischemia time on DCD liver transplant recipient outcomes. Clin Transplant 2017; 31: e13068-e13070.
- Ho KJ, Owens CD, Johnson SR, et al. Donor postextubation hypotension and age correlate with outcome after donation after cardiac death transplantation. Transplantation 2008;85(11):1588-94.
- Boteon APCS, Schlegel A, Kalisvaart M, et al. Retrieval practice or overall donor and recipient risk: what impacts on outcomes after donation after circulatory death liver transplantation in the United Kingdom? Liver Transpl 2019;25:545–58.
- Schlegel A, Kalisvaart M, Scalera I, et al. The UK DCD risk score: a new proposal to define futility in donation after circulatory death liver transplantation. J Hepatol 2018;68:456-64.
- Hertl M, Howard T, Lowell J, et al. Changes in liver core temperature during preservation and rewarming in human and porcine liver allografts. Liver Transpl 1996;2:111-7.
- Farid SG, Attia MS, Vijayanand D, et al. Impact of donor hepatectomy time during organ procurement in donation after circulatory death liver transplantation: the United Kingdom experience. Transplantation 2019;103:e79–e88.
- Goussous N, Alvarez-Casas J, Dawany N, et al. Ischemic cholangiopathy postdonation after circulatory death liver transplantation: donor hepatectomy time matters. Transplant Direct 2022;8(1):e1277.
- Jochmans I, Fieuws S, Tieken I, et al. The impact of hepatectomy time of the liver graft on post-transplant outcome: a Eurotransplant cohort study. Ann Surg 2019;269:712–7.
- Gilbo N, Fieuws S, Meurisse N, et al. Donor hepatectomy and implantation time are associated with early complications after liver transplantation. Transplantation 2021;105:1030–8.
- Van Leeuwen OB, van Reeven M, van der Helm D, et al. Donor hepatectomy time influences ischemia-reperfusion injury of the biliary tree in donation after circulatory death liver transplantation. Surgery 2020;168:160–6.
- Nostedt J, Skubleny D, Shapiro AMJ, Campbell S, et al. Normothermic ex vivo machine perfusion for liver grafts recovered from donors after circulatory death: a systematic review and meta-analysis. HPB Surgery 2018; 6867986.
- Nasralla D, Coussious CC, Mergental H, et al. A randomized clinical trial of group normothermic preservation in liver transplantation. Nature 2018;557:50-6.
- MacConmara M, Hanish S, Hwang C, et al. Making every liver count. Increased transplant yield of donor livers through normothermic machine perfusion. Ann Surg 2020;272:397-401.
- Schlegel A, Kron P, De Oliveira M, et al. Is single portal vein approach sufficient for hypothermic machine perfusion of DCD liver grafts? J Hepatol 2016;41:239-41.
- Van Rijn R, Schurink IJ, de Vries Y, et al. Hypothermic machine perfusion in liver transplantation—a randomized trial. N Engl J Med 2021;384:1391-1401.
- Dondossola D, Ravaioli M, Lonati C, et al. The role of ex situ hypothermic oxygenated machine perfusion and cold preservation time in extended criteria donation after circulatory death and donation after brain death. Liver Transpl 2021;27:1130–43.
- Muller X, Mohkam K, Mueller M, et al. Hypothermic oxygenated perfusion versus normothermic regional perfusion in liver transplantation from controlled donation after circulatory death: first international comparative study. Ann Surg 2020;272:751-8.
- De Carlis R, Schlegel A, Frassoni S, et al. How to preserve liver grafts from circulatory death with long warm ischemia? A retrospective Italian cohort study with normothermic regional perfusion and hypothermic oxygenated perfusion. Transplantation 2021;105(11):2385-96.
- Martins PN, Rizzari MD, Ghinolfi D, et al. Design, analysis, and pitfalls of clinical trials using ex situ liver machine perfusion: the International Liver Transplantation Society Consensus Guidelines. Transplantation. 2021;105:796–815.
- Gulsem M, Girotra M, Cengiz G, et al. HTK preservation solution is associated with increased biliary complications among patients receiving DCD liver transplant: a single center experience. Ann Transplant 2013;18:69-75.
- Adam R, Delvart V, Karam V, et al. Compared efficacy of preservation solutions in liver transplantation: a long-term graft outcome study from the European Liver Transplant Registry. Am J Transplant 2015;15:395–406.
- Adam R, Cailliez V, Karam V. Evaluation of HTK preservation solutions in liver transplantation: a long-term propensity-based analysis of outcome from the European Liver Transplant Registry. Am J Transplant 2017;17:585–86.
- Hameed AM, Pang T, Yoon P, et al. Aortic versus dual perfusion for retrieval of the liver after brain death: a national registry analysis. Liver Transpl 2018;24:1536–44.
- Ghinolfi D, Tincani G, Rreka E, et al. Dual aortic and portal perfusion at procurement prevents ischaemic-type biliary lesions in liver transplantation when using octogenarian donors: a retrospective cohort study. Transpl Int 2019 Feb;32(2):193-205.
- Oniscu GC, Mehew J, Butler AJ, et al. Improved organ utilization and better transplant outcomes with in situ normothermic regional perfusion in controlled donation after circulatory death. Transplantation. 2023 Feb 1;107(2):438-48.
- Maheshwari A, Maley W, Li Z, Thuluvath PJ. Biliary complications and outcomes of liver transplantation from donors after cardiac death. Liver Transplant 2007;13:1645–53.
- de Vera ME, Lopez-Solis R, Dvorchik I, et al. Liver transplantation using donation after cardiac death donors: long- term follow-up from a single center. Am J Transplant 2009;9:773–81.
- Deoliveira ML, Jassem W, Valente R, et al. Biliary complications after liver transplantation using grafts from donors after cardiac death: Results from a matched control study in a single large volume center. Ann Surg 2011;254:716– 22.
- Bartlett A, Vara R, Muiesan P, et al. A single center experience of donation after cardiac death liver transplantation in pediatric recipients. Pediatr Transplant 2010;14:388–92.
- Gozzini S, Perera T, Mayer D, et al. Liver transplantation in children using non-heart-beating donors (NHBD). Pediatr Transplant 2010;14:554–7.
- Van Rijn R, Hoogland PER, Lehner F, et al. Long-term results after transplantation of paediatric liver grafts from donation after circulatory death donors. PLoS One 2017; 12: e0175097.
- Werner MJ, van Leeuwen OB, de Jong IEM, et al. First report of successful transplantation of a pediatric donor liver graft after hypothermic machine perfusion. Pediatr Transplant 2019;23:e13362.
- Thorne AM, Lantinga V, Bodewes S, et al. Ex situ dual hypothermic oxygenated machine perfusion for human split liver transplantation. Transplant Direct 2021;7:e666.
- Mabrut JY, Lesurtel M, Muller X, et al. Ex vivo liver splitting and hypothermic oxygenated machine perfusion: technical refinements of a promising preservation strategy in split liver transplantation. Transplantation 2021;105:e89-e90.
- Brockmann JG, Vogel T, Coussios C, et al. Liver splitting during normothermic organ preservation. Liver Transplant 2017; 23:701-6.
- Stephenson BTF, Bonney GK, Laing RW, et al. Proof of concept: liver splitting during normothermic machine perfusion. J Surg Case Reports 2018:3:1-6.
11. Pancreas
We recommend that:
- Pancreas transplantation from DCD donors offers similar outcomes to DBD donors, in terms of graft and patient survival, therefore DCD donors should be considered an acceptable source of pancreatic grafts. (1B)
- Outcomes of DCD pancreas transplants are better with lower cold ischaemic times and ideally this should be kept to within 10 hours. (1B)
- Although DCD organs can be used for solitary pancreas transplants in pancreas transplant alone or pancreas after kidney, numbers are limited, and therefore most evidence supports their use for simultaneous pancreas and kidney transplantation. (1C)
- Although arterial and venous thrombosis rates are similar between DCD and DBD pancreata, appropriate systemic anticoagulation protocols should be considered. (1C)
We suggest that:
- Pancreas transplants from DCD donors are at increased risk of reperfusion pancreatitis and this may be exacerbated by prolonged cold ischaemia time >12 hours and increasing donor age >55 years. Ideal donors should be <45 years old and have a BMI <28 kg/m2. (2C)
- The pancreas team should stand down after a functional warm ischaemia time (systolic BP <50 mmHg) of 60 minutes, unless the pancreas is recovered using NRP, which may allow prolonged warm times. (2C)
- There is limited evidence regarding the effect of recipient risk factors in terms of outcome after DCD pancreas transplantation, however, we would suggest considering the same risk factors as those that may contribute to an adverse outcome after DBD pancreas transplants (e.g., higher recipient BMI, cardiovascular morbidity, and technical surgical factors). (2C)
11.1 Introduction
DCD donors are an acceptable and established source of pancreatic grafts for transplantation. Worldwide, the number of DCD pancreas transplants remains low compared to DBDs. However, pancreas transplant centres in the UK have built up a volume of expertise with DCD pancreas transplantation. As with other organs, cold ischaemia is detrimental to the outcome of DCD pancreas transplantation in proportion to its duration. As such, every effort should be made to keep cold ischaemia time as short as possible to ensure optimal outcomes.
11.2 Donor Selection
The selection of DCD donors for pancreas transplantation has been more restrictive than that for DBD donors. A previous review of the UK experience reported that DCD donors tended to be younger (median age 28 vs 37 years, p<0.001), had a lower BMI (23 vs 24 kg/m2, p=0.04), and less commonly had a cerebrovascular cause of death (33 vs 60%, p<0.0001) (1). A more recent UK registry analysis of 2269 simultaneous pancreas-kidney (SPK) transplants (18.1%; 403 from DCD donors) showed similar baseline donor characteristics, but a significant difference in pancreas cold ischaemic time (median 634 vs 685 min) in favour of DCD transplants (2). Admittedly, expected pancreas cold ischaemic time (CIT) remains a major factor involved in decision-making at the time of a DCD pancreas offer and is largely influenced by the availability of virtual cross-match, travel time to recipient centres and local arrangements of surgical teams.
The number of PTA or PAK transplants from DCD donors is much smaller, and does not allow a rigorous analysis of factors affecting donor selection, but the following broad comments reflect current experience and practice in the UK with SPK transplants, which represent 90% of pancreas transplant activity. UK data suggest a poorer outcome for DCD solitary pancreas transplantation (1).
Age
UK transplant registry data suggest that increasing donor age remains a significant factor contributing to transplant outcomes for DCD pancreata in the UK. Current criteria are to consider all potential donors up to the age of 60 years, although an increased risk of pancreas graft loss should be taken into account when selecting donors older than 50 years (HR 2.28, 95%CI 1.60-3.26; p<0.01) (2).
Body mass index
A low donor BMI (<28 kg/m2) is preferred, but potential donors with higher BMI should still be referred and organs should be considered for acceptance when the donor BMI is ≤30 kg/m2. Pancreata from donors with a BMI >30 kg/m2 should be referred for consideration of islet transplantation, as per the UK national pancreas offering scheme.
Time post-withdrawal
A prolonged functional warm ischaemic time (FWIT), from the point at which the systolic pressure is ≤50 mmHg for 60 minutes, is a reasonable indication to abandon pancreas retrieval. Otherwise, a donor in whom the blood pressure is stable may still yield a transplantable pancreas sometime after treatment withdrawal. In general, the retrieval team should be prepared to retrieve the pancreas for up to three hours following the withdrawal of support. The decision to stand down sooner should be made on the basis of the blood pressure profile and after consideration of other potentially adverse donor criteria. Pancreata from donors with a withdrawal period of over 60 minutes have been used for transplantation successfully (1,3).
Inotropes
Although high doses of inotropes are generally agreed to be detrimental, there is no good evidence on which to base national criteria. Inotrope levels should not, therefore, be used to exclude the referral of DCDs for pancreas retrieval.
Amylase and glucose levels
There is no good evidence that the level of either amylase or glucose has prognostic significance, and these should not be used to exclude either the referral or the transplantation of DCD pancreata. HbA1c may be considered if there are concerns about hyperglycaemia. Further research is needed to understand how to best use information on donor insulin use when selecting donor pancreases for islet or pancreas transplantation.
Steatosis and fibrosis
These factors are largely subjective and difficult to quantify, and the precise significance of differing degrees of steatosis or fibrosis are uncertain. For these reasons, retrieval should proceed unless the changes are obvious. It is important that the transplanting surgeon should be able to speak to the retrieving surgeon to discuss the quality of the donor organs. Photography of the pancreatic graft on the back bench is also recommended as a useful means of communication.
Caution
Suggested donor criteria for pancreas transplantation are shown in Table 11.1., based on rapid recovery. However, this is an evolving field, and some of these variables may alter if the pancreas is recovered using NRP. It is important to note that transplanting centres in the UK will have built up a volume of expertise with extended criteria donor (ECD) DCD criteria, and these criteria should not restrict innovation.
Table 11.1 Donor criteria for pancreas transplantation.
| Donor variables | Ideal DCD | Marginal (ECD) DCD |
| Age (years) | < 45 | 45-60 |
| BMI (kg/m2) | < 28 | 28-30 |
| FWIT (min) | ≤ 30 | > 30 |
| Expected CIT (hr) | ≤ 10 | > 10 |
| Steatosis | None | Mild-moderate |
| Recommendation | All potential pancreas donors fulfilling these criteria should be used | These grafts should be considered after careful assessment |
11.3 Organ Preservation
Warm ischaemia time
The endocrine function of the pancreas appears to tolerate warm ischaemia relatively well, but the complication of reperfusion pancreatitis has serious implications in terms of both morbidity and graft survival and this is in part related to the duration of warm ischaemia. A withdrawal to perfusion time (defined as the time from donor treatment withdrawal to initiation of cold perfusion or the start of NRP, see Chapter 3) of less than 30 minutes is preferred, but organs with a withdrawal to perfusion time of more than 30 minutes should be considered. In the UK, the median DCD pancreas withdrawal to perfusion time (duration between withdrawal of life support treatment and perfusion with cold preservation fluid) is 26 min (IQR 22-31), with a median time between WLST and circulatory arrest of 14 min (IQR 9-18) (2).
In situ cold perfusion and preservation
In situ cold perfusion for pancreas donors should be via the aorta only (with additional perfusion of the liver via the portal vein without impeding venous drainage from the pancreas by allowing free drainage of the portal vein proximal to the portal infusion cannula).
Thrombolysis
There is weak evidence for the benefit of an initial streptokinase flush that comes from DCD kidney retrieval. There is no equivalent evidence in DCD pancreas transplantation.
Cold ischaemia time
As with other organs, there is good evidence that cold ischaemia is detrimental to the outcome of pancreas transplantation in proportion to its duration. CIT remains one of the main predictors of pancreas graft failure. UK data suggest that, when DBD and DCD grafts are combined, a CIT of >12 hours is associated with a significantly increased risk of graft loss (HR 1.80, 95% CI 1.04-3.07) (2). A shorter CIT (ideally up to 10 hours) may be more appropriate for DCD SPKs, though the previous analysis was unable to identify a CIT beyond which DCD donor pancreas graft survival deteriorates.
11.4 Organ Quality Assessment
There is no ‘standard’ quality assessment. Assessment is largely based on visual inspection and clinical experience. In general, a quality assessment can be made by looking at the quality of perfusion of the gland and duodenum, as well as the amount of fat infiltration, and by feeling the texture of the gland for fibrosis. Another important clinical variable is the presence of pancreas damage (vascular, capsular or parenchymal), which accounts for a significant proportion of retrieved but non-utilised grafts (4). These observations should be considered alongside the donor characteristics, haemodynamics and duration of the withdrawal period. Interestingly, in the UK the non-utilisation rates between retrieved DBD and DCD pancreata are very similar, albeit still quite high, reflecting similar attitudes of clinicians towards different types of donors (4).
11.5 Recipient Selection
There is relatively little reported experience of DCD pancreas transplantation to guide recipient selection (see also section 11.8). Recipient selection should be the same as for DBD pancreas transplantation as supported by national registry analyses. National databases do not show significant differences in recipient characteristics between the two groups. Therefore, the indications for DCD pancreas transplantation are no different to those for pancreas transplantation in general and are summarised in the national policy (POL185/6) at https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/17326/pancreas-selection-policy-pol185.pdf. However, the UK data do suggest a poorer outcome for DCD solitary pancreas transplantation (1).
11.6 Organ Allocation
As with other organs, there is good evidence that cold ischaemia is detrimental to the outcome of pancreas transplantation in proportion to its duration. As mentioned above, pancreas cold ischaemia time should be kept reasonably short to ensure optimal outcomes, balancing this biological requirement with the need to maintain equity of access to transplantation. Current allocation arrangements in the UK are summarised on the NHSBT website at https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/26593/pol199.pdf. The current UK DCD pancreas allocation scheme aims to minimise the distance the pancreas travels to a recipient centre, though this is being reviewed in the light of recent evidence (2).
11.7 Immunosuppression
There are no data that indicate an optimal immunosuppressive regimen for a DCD pancreas. The immunosuppressive requirements of pancreas transplantation appear to be greater than those of kidney transplantation. Consideration of a kidney-friendly (i.e., low-dose tacrolimus) protocol seems appropriate, with an induction agent such as basiliximab or alemtuzumab followed by mycophenolate and an initial low-dose tacrolimus regimen. Although local immunosuppression protocols will probably continue to vary based on clinical experience and acceptable clinical rejection rates, most UK units currently use alemtuzumab for induction for both DBD and DCD pancreata, whether it is for SPKs or solitary pancreas transplantation. Single-dose alemtuzumab is equally effective as the more commonly used two-dose regimen, as shown by similar pancreas graft rejection rates and survival, and also has the potential to reduce viral and systemic infection rates post-transplant, as suggested by published evidence (5,6).
11.8 Outcomes
Over the last decade in the UK, pancreatic transplantation from DCD donors has been performed at relatively steady numbers of around 50 per year, comprising roughly a quarter of the total number of pancreatic transplants (3). Worldwide, the number of DCD pancreas transplants remains low compared to DBDs.
Most of the published outcome data come from the USA and the UK. These suggest that outcomes from DCD SPK transplantation are comparable to those of DBD SPK transplantation. The results of pancreas-alone transplantation are worse than those of SPK transplantation. However, the outcomes after DCD pancreas-alone transplantation are not significantly worse than that of DBD pancreas-alone transplantation (1).
Early reports from DCD pancreata raised concerns regarding pancreas warm and cold ischaemic times and subsequent graft thrombosis, implying that DCD pancreas transplantation leads to suboptimal outcomes. High graft failure rates within the first-year post-transplant have been reported in relatively small cohorts, and DCD donor status was considered an independent risk factor for pancreas graft loss in a risk-adjusted analysis from the US in 2010 (7). Inevitably, DCD pancreas transplants have been reluctantly performed over the last decade, and almost exclusively in high-volume centres around the world.
Significant experience has been accumulated in the UK with DCD SPK transplantation since the early 2000s. A recent national registry analysis from 2005-2018 included 1825 DBD and 403 DCD SPK transplants (2), the largest pancreas DCD cohort published to date. Important differences were noted in the baseline characteristics of DCD versus DBD transplants. DCD donors were younger, slimmer, less likely to have cerebrovascular accident as the cause of death and with lower terminal creatinine compared to DBD donors. Recipients of DCD SPKs were more likely to have a shorter waiting time on the list, had shorter pancreas CIT, were more likely to receive lymphocyte depletion at induction and were transplanted in a more recent era. These findings suggest a degree of donor and recipient selection bias in the context of DCD pancreas transplantation, although risk-adjusted analyses failed to show any substantial differences in clinical outcomes. Five-year pancreas and kidney graft survival were similar between the two groups (Figure 11.1), with higher rates of kidney DGF as expected. Five-year patient survival did not differ.
Among clinically relevant risk factors examined in a multivariate survival model for the whole SPK cohort, increasing donor age, pancreas CIT and recipient BMI were found to be significant predictors of death-censored graft loss. Although donor age and pancreas CIT were not associated with reduced pancreas graft survival in the DCD subgroup, this should be interpreted with caution: the median CIT in the UK is 10.5 hours, much shorter than reports from other countries, and very few pancreata from DCD donors older than 50 have been utilised. From a practical perspective, DCD SPKs appear to have comparable outcomes to DBDs, provided pancreas CIT and donor age remain within reasonable limits. Despite the higher rate of kidney DGF in recipients of organs from DCD donors, longer-term kidney graft function and survival are at least equivalent to those from DBD donors (chapter 9).
A recent systematic review and meta-analysis compared DCD and DBD pancreas transplants and confirmed the absence of significant differences in terms of graft and patient survival up to ten years post-transplant (8). Although the recipients of DCD grafts showed higher rates of pancreas thrombosis (OR 1.67; 95%CI 1.04-2.67), subgroup analysis revealed that donor treatment with antemortem heparin administration was protective from graft thrombosis. Nevertheless, graft thrombosis in DCD pancreata was not thoroughly investigated in correlation with other clinical factors such as CIT, WIT, donor age etc, possibly due to methodological flaws and a lack of relevant data. This meta-analysis did not include the most recent UK analysis (the largest in the world), and therefore needs to be interpreted cautiously.
In the recent UK national registry analysis (2), early graft survival appeared nearly identical between DBD and DCD organs. Of those pancreatic grafts that failed within the first year of transplantation, the rates of arterial or venous thromboses were similar between the two groups. In any case, we recommend careful evaluation of DCD donors and appropriate pharmacological thromboprophylaxis in the post-transplant period to reduce the incidence of graft thrombosis, according to local policies.
Figure 11.1 Death-censored pancreas graft survival for simultaneous pancreas– kidney (SPK) transplant recipients in the UK (2005– 2018), by donor type (2).
In the UK, risk-adjusted patient and graft survival rates are similar between DBD and DCD SPK recipients. A summary of recent data is shown below (Table 11.2). The small number of pancreas-only transplants performed in the UK and worldwide do not allow risk adjustment, but survival rates remain similar between DBD and DCD transplants. Unadjusted pancreas-only survival rates are also shown in Table 11.2 (3).
Table 11.2 Risk-adjusted (SPK) and unadjusted pancreas-only patient and pancreas graft survival, NHSBT data (2016-2020). NB: five-year survival rates also include transplants from 2012-2016.
| Type of transplant | N | 1-yr patient survival (%); 95% CI | 5-yr patient survival (%); 95% CI | 1-yr pancreas survival (%); 95% CI | 5-yr pancreas survival (%); 95% CI |
| First SPK | 621 | 99 (97-99) | 89 (86-91) | 93 (90-95) | 80 (77-83) |
| Pancreas only | 35 | 100 (n/a) | 84 (68-93) | 88 (70-95) | 56 (44-66) |
11.9 Novel Techniques in Pancreas Transplantation
Normothermic regional perfusion (NRP) has been a major development in recent years (9). While NRP shows promising improvements in DCD liver transplants, clinical outcomes in pancreas transplantation have not been adequately investigated. The technical feasibility of this technique followed by the successful transplantation of abdominal organs was demonstrated in a landmark UK study, which included two cases of SPK transplants with primary kidney and pancreatic function without adverse events (10). Experience from a single centre, comparing standard DCD vs NRP DCD pancreata, showed that NRP provides at least comparable outcomes (11). This might be due to the careful selection of DCD donors who proceed to pancreas transplantation, where substantial benefits in clinical outcomes would be harder to obtain. Whether NRP could allow better utilisation of more “marginal” DCD pancreata remains to be seen. It is hoped this question will be answered with future studies.
As yet, there is no evidence to support the routine use of either ex situ hypothermic (12-14) or normothermic machine perfusion for pancreata (15). Preliminary studies only describe experimental techniques but to our knowledge, a clinical transplant has never been performed.
The evidence summarised above demonstrates comparable outcomes of pancreas transplantation betweenDBD and DCD donor organs. However, we acknowledge the inevitable donor and recipient selection bias, which inherently influences clinical decision-making. Within these limits, DCD pancreas transplantation is considered equally safe and effective as DBD pancreas transplantation in the UK. The accumulation of experience with these donors, along with further use of machine perfusion techniques, may allow further expansion of this valuable donor pool in the near future and the design of prospective clinical studies.
References
- Muthusamy AS, Mumford L, Hudson A, et al. Pancreas transplantation from donors after circulatory death from the United Kingdom. Am J Transplant 2012;12: 2150-6.
- Callaghan CJ, Ibrahim M, Counter C, et al. Outcomes after simultaneous pancreas-kidney transplantation from donation after circulatory death donors: A UK registry analysis. Am J Transplant. 2021 Nov;21(11):3673-83.
- Annual report on pancreas and islet transplantation (NHSBT) (https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/24590/final-nhsbt-pancreas-and-islet-transplantation-annual-report-2020-2021.pdf)
- Cornateanu SM, O’Neill S, Dholakia S, et al. Pancreas utilization rates in the UK – an 11-year analysis. Transplant Int 2021;34:1306-18.
- Kaufman DB, Leventhal JR, Gallon LG, et al. Alemtuzumab induction and prednisone-free maintenance immunotherapy in simultaneous pancreas-kidney transplantation comparison with rabbit antithymocyte globulin induction – long-term results. Am J Transplant 2006;6:331-9.
- Magliocca JF, Odorico JS, Pirsch JD, et al. A comparison of alemtuzumab with basiliximab induction in simultaneous pancreas-kidney transplantation. Am J Transplant 2008;8:1702-10.
- Axelrod DA, Sung RS, Meyer KH, et al. Systematic evaluation of pancreas allograft quality, outcomes and geographic variation in utilization. Am J Transplant 2010;10(4):837-45.
- Shahrestani S, Webster AC, Lam VWT, et al. Outcomes from pancreatic transplantation in donation after cardiac death. Transplantation 2017;101:122-30.
- Butler AJ, Randle LV, Watson CJ. Normothermic regional perfusion for donation after circulatory death without prior heparinization. Transplantation 2014;97:1272-78.
- Oniscu GC, Randle LV, Muiesan P, et al. In situ normothermic regional perfusion for controlled donation after circulatory death—the United Kingdom experience. Am J Transplant 2014;14:2846-54.
- Richards JA, Roberts JL, Fedotovs A, et al. Outcomes for circulatory death and brainstem death pancreas transplantation with or without use of normothermic regional perfusion. Br J Surg 2021;108(12):1406-8.
- Hamaoui K, Gowers S, Sandhu B, et al. Development of pancreatic machine perfusion: translational steps from porcine to human models. J Surg Res 2018 Mar;223:263-74.
- Branchereau J, Renaudin K, Kervella D, et al. Hypothermic pulsatile perfusion of human pancreas: preliminary technical feasibility study based on histology. Cryobiology 2018 Dec;85:56-62.
- Leemkuil M, Lier G, Engelse MA, et al. Hypothermic Oxygenated Machine Perfusion of the Human Donor Pancreas. Transplant Direct. 2018 Sep 7;4(10):e388.
- Barlow AD, Hamed MO, Mallon DH, et al. Use of ex vivo normothermic perfusion for quality assessment of discarded human donor pancreases. Am J Transplant. 2015 Sep;15(9):2475-82.
12. Islets
We suggest that:
- Selection criteria for recipients of islets from DCD donors should be the same as for DBD donors. (2B)
- A pancreas recovered from a DCD donor should be allocated for islet isolation through the National Pancreas Offering Scheme. (2B)
- Satisfactory functional islet preparations can be routinely obtained from DCD donors and are as functional in vitro and after clinical transplantation as DBD islets. (2B)
- There is no difference in the long-term outcome of islet transplants from DCD donors when compared to a DBD donor although the comparative cohort is small. (2C)
12.1 Introduction
There is relatively limited world-wide clinical experience of islet transplants recovered from DCD donors. Furthermore, there is limited information as to what constitutes an ideal DCD pancreas for islet transplantation. Given the above, ideal donor criteria have to be extrapolated from the ideal DBD donor as recommended by the National Clinical Guideline islet transplant program. Younger donors yield fewer islets after islet isolation because of the effect of collagenase on the pancreatic extracellular matrix (1,2), although there is evidence to suggest overall functional outcome is better in younger donors (see outcomes below). The smaller the donor weight, the smaller the donor pancreas and thus fewer islets are isolated for transplantation (3-9). Obese non-diabetic donors are excellent for islet isolation because of a higher beta-cell mass. Human islets are very intolerant to prolonged periods of both warm and cold ischaemia and this is crucial to successful islet transplantation (10).
12.2 Donor Selection
The following summarises the recommendations for DCD donor selection in the UK:
Standard Criteria Donors (SCD)
- Age 18-45 yr
- Weight 60-100 kg
- BMI 21-35 kg/m2
- Cold ischaemia <8 hr
- FWIT <30 min
Extended Criteria Donors (ECD)
- Age <18 yr or >50 yr
- Weight 40-60 or >100 kg
- BMI 36-40 kg/m2
- Cold ischaemia >8 hrs
- FWIT 30-60 min
- Retrieval damage (parenchymal/duct transection/traumatic capsular damage)
Contraindications*
- Age >65 yr
- BMI >40 kg/m2
- Cold ischaemia > 12 hrs
- FWIT >60 min
- Diabetes mellitus
- Evidence of pancreatic disease (e.g., chronic pancreatitis)
- Positive for HCV, HIV, HBV
- Variant CJD
- Untreated systemic infection
- Malignancy, myeloma, lymphoma, leukaemia
- Invasive cancer in the last 3 years, excluding non-melanoma skin cancer and primary brain tumour
*Absolute contraindications are generally the same as those advised by NHSBT and those outlined in SaBTO guidelines
Allocation
Organs for islet isolation and transplantation should be allocated through the National Pancreas Offering Scheme. Current allocation arrangements in the UK may be found on the NHSBT website http://www.organdonation.nhs.uk/about_transplants/organ_allocation/pancreas/.
12.3 Donor Warm Ischaemic Times
Following withdrawal of treatment there is FWIT when the donor’s systolic BP < 50mmHg (see Chapter 3) and an asystolic time following cardiac arrest. Asystolic time can vary in the UK depending on whether treatment is withdrawn in the ITU setting or in the operating theatre. It is recommended that FWIT is kept below 30 minutes. The Leiden group recently published their outcomes from 126 DCD category 3 islet isolations (11). The average FWIT was 23.2 minutes (+/- 6.4 SD) and although islet yields were slightly lower when compared to DBD donors, functional outcomes following transplantation were no different. After multivariate analysis no significant correlations were found between different warm ischemia periods and islet yield within their data set (FWIT range approximately 10-40 minutes). The Belgian group analysed 141 DCD category 3 islet isolations and again found no overall correlation between islet yield and total WIT but found that asystolic times greater than 10 minutes were associated with lower yields and poorer function (measured by insulin content) (12).
12.4 Organ retrieval and organ preservation
Human islets are very intolerant to prolonged periods of both warm and cold ischaemia. It is important that pancreas retrieval for islet transplantation is performed to the same high standard as for whole pancreas transplantation, cooling the donor organs as rapidly as possible during the retrieval process. As with pancreases for solid organ transplantation, in situ perfusion with preservation fluid should be via the aorta only (with additional perfusion of the liver via the portal vein being instituted by opening the portal vein without impeding venous drainage from the pancreas).
Care should be taken to avoid capsular and parenchymal damage and haematomas as they can adversely affect the islet isolation procedure and success especially if damage is extensive. If there is aberrant anatomy that precludes the use of the pancreas for solid organ transplant, the pancreas can still be used for islets providing the pancreas has not been damaged during the recovery process. If the pancreas is to be used for islet isolation extra vessels are not required.
NRP is increasingly used for DCD donors in the UK (see Chapter 7) and is discussed below.
12.5 In Situ and Ex Situ Machine Perfusion
Over the last decade there has been an increase in the use of ex situ machine perfusion of transplant organs (cold, cold oxygenated and warm oxygenated). Pancreas perfusion is still largely experimental and to our knowledge there have not been any clinical transplants performed after ex situ machine perfusion. NRP is increasingly used in Europe with improved outcomes in liver and kidney transplantation and increased organ utilisation for pancreas transplantation (13). The effect of NRP on pancreas and islet transplant outcomes is less clear but preliminary data in the UK and Europe is encouraging with successful islet transplants having been performed. As NRP allows monitoring of physiological and biochemical parameters during perfusion then it may be possible to use these markers (e.g., lactate, ALT, LDH, amylase) for donor selection. However, data are currently insufficient to enable guidance.
12.6 Organ Quality Assessment
The same quality criteria apply as described in solid organ pancreas transplantation (Chapter 11).
12.7 Recipient Selection
Selection of recipients for islet transplantation from a DCD donor should be the same as for those from DBD donors:
- Insulin-sensitive patients with Type I diabetes and normal renal function who experience recurrent severe hypoglycaemia despite optimised specialist management.
- Insulin-sensitive patients with a renal allograft who are unable to maintain HbA1c <7.0% (53 mmol/mmol) despite optimised specialist management.
There is no evidence that these groups of patients are disadvantaged by receiving islet transplants from a DCD pancreas. Those patients waiting for prolonged periods for second infusions are automatically given priority through the National Pancreas Offering Scheme.
12.8 Immunosuppression
There are no data to indicate an optimal immunosuppressive regimen for DCD islet transplants. The current practice for the small number performed in the UK includes an induction agent such as alemtuzumab or basiliximab, followed by a mycophenolate- and tacrolimus-based maintenance regimen.
12.9 Outcomes
The first islet transplant from a DCD donor was described in 2003 (14) and following this a number of clinical studies have originated from Japan (5,8,15). Although insulin independence from a single islet infusion from a DCD donor has been described, the Japanese data only describe 3/64 patients achieving insulin independence, which is significantly lower than would be expected from DBD islet preparations. More recently, a series of DCD islet transplants from the Netherlands has demonstrated that although DCD donors resulted in lower yield compared to DBD donors (395,000 vs 480,000) there was no demonstrable difference in overall function (AUC C-peptide) during mixed meal tolerance tests and Igls scores (11).
In the UK, data from the King’s group have demonstrated that satisfactory functional islet preparations can be routinely obtained from DCD donors (9). A recent analysis by NHSBT demonstrated that out of 314 islet transplants performed in the UK, 39 have been from DCD donors and there was no statistically significant difference in one-year graft outcome between first routine islet transplants from DCD donors when compared to DBD donors (unpublished data).
Factors that appear to predict good clinical outcomes are: asystolic time <10 minutes; FWIT <30 minutes; short CIT; and donor age <55 years. There is, however limited data on the long-term outcomes of islet transplants from DCD donors.
References
- Balamurugan An, Chang Y, Bertera S, et al. Suitability of human juvenile pancreatic islets for clinical use. Diabetologia 2006;49:1845-54.
- Sung-Hee I, Matsumoto I, Sawad T, et al. Effect of donor age on the function of isolated human islets. Diabetes 2006;55:1361-8.
- Lakey JRT, Warnock GL, Rajotte RV, et al. Variables in organ donors that affect the recovery of human islets of Langerhans. Transplantation 1996;61:1047-53.
- Kin T, Murdoch TB, Shapiro AM, et al. Estimation of pancreas weight from donor variables. Cell Transplant 2006;15:181-5.
- Liu X, Matsumoto S, Okitsu T, et al. Analysis of donor and isolation variables from NHBD using Kyoto islet isolation method. Cell Transplant 2008;17:649-56.
- Matsumoto I, Sawada T, Nakano M, et al. Improvement in islet yield from obese donors for human islet transplants. Transplantation 2004;78:880-5.
- Ridgway D, Manas D, Shaw J, et al. Preservation of the donor pancreas for whole pancreas and islet transplantation. Clin Transplant 2010;24:1-19.
- Saito T, Gotoh M, Satomi S, et al. Islet transplantation using donors after cardiac death: report of the Japan Islet Transplantation Registry. Transplantation 2010;90:740-7.
- Zhao M, Muiesan P, Amiel SA, et al. Human islets derived from donors after cardiac death are fully biofunctional. Am J Transplant 2007;7:2318-25.
- Lakey JR, Kneteman NM, Rajotte RV, et al. Effect of core pancreas temperature during cadaveric procurement on human islet isolation and functional viability. Transplantation 2002;73:1106-10.
- Doppenberg JB, Nijhoff MF, Engelse MA, et al. Clinical use of donation after circulatory death pancreas for islet transplantation. Am J Transplant. 2021;00:1-11. https://doi.org/10.1111/ajt.16533
- De Paep DL, Van Hulle F, Ling Z, et al. Lower beta cell yield from donor pancreases after controlled circulatory death prevented by shortening acirculatory warm ischemia time and by using IGL-1 cold preservation solution. PLoS ONE 2021;16(5): e0251055. https://doi.org/10.1371/journal.pone.0251055
- Oniscu GC, Mehew J, Butler AJ, et al. Improved organ utilization and better transplant outcomes with in situ normothermic regional perfusion in controlled donation after circulatory death. Transplantation 2022 Aug 22.
- Markmann JF, Deng S, Desai NM, et al. The use of non-heart-beating donors for isolated pancreatic islet transplantation. Transplantation 2003;75:1423-9.
- Kenmochi T, Maruyama M, Saigo K, et al. Successful islet transplantation from the pancreata of non-heart beating donors. Transplant Proc 2008;40:2568-70.
13. Lung
We recommend that:
- DCD lungs should not be regarded as extended or marginal. Transplant survival and outcomes are at least similar to DBD organs. (1B)
- Pre-transplant ex vivo lung perfusion (EVLP) is advised in case of uncertain graft performance to safely extend donor and procedural criteria (long warm ischaemia, poor flush, clots). (1B)
- All patients on the lung transplant waiting list have the potential to receive DCD lungs. (1C)
We suggest that:
- The donor selection criteria for DCD lung transplantation should be the same as for DBD. (2B)
- An antegrade and retrograde flush perfusion should be performed at the time of lung procurement. (2B)
- Acceptance criteria on EVLP include measures of pulmonary compliance, vascular resistance, and gas exchange. (2C)
- If available, the use of EVLP as part of the procurement process should be considered. (2D)
13.1 Introduction
Transplantation of lungs from Category III DCD donors is an established practice in a number of centres around the world with recipient outcomes comparable to transplantation of lungs from DBD donors. Static cold storage is the standard technique for lung preservation in most centres but the use of ex vivo lung perfusion (EVLP) can enable warm viability testing of donor lungs and is associated with excellent recipient outcomes.
13.2 Donor Selection
All DCD organ donors age <65 years who do not have any of the contraindications listed below are suitable to donate lungs:
- Chest trauma with extensive bilateral lung contusions
- Convincing radiological evidence of bilateral pneumonic consolidation
- Pre-existing structural lung changes (e.g., emphysema or multiple large bullae)
- Previous complex intra-pleural thoracic surgery or dense adhesions prohibiting safe procurement
- Systemic arterial PO2 <30 kPa on 100% FiO2 and 5 cmH2O PEEP, or equivalent FiO2: PaO2 within 12 hours
- Bronchoscopy (if available) showing inflammation/soiling of the airway, and recurrent secretions in the distal airway after adequate toilet
- Sustained peak airway pressure >30 cmH2O
If EVLP is not being considered, the following are also required:
- The proposed transplant satisfies the criteria as for standard DBD donor lungs (if information is available)
- DCD donor from modified Maastricht Category III or IV
- Systemic arterial PO2 >40 kPa on 100% FiO2 and 8 cmH2O PEEP, or equivalent FiO2: PaO2 within previous 12 hour
- Functional warm ischaemic time (FWIT) <30 minutes (FWIT starts when donor systolic BP <50 mmHg)
- Withdrawal time (i.e., cessation of life support to asystole) <120 minutes
13.2.1 Information on Referral
Information should be available as per the ‘Organ Donation after Circulatory Death: A Consensus Statement from the British Transplantation Society and Intensive Care Society’ (1).
For lung donation, the following additional donor information should be available at the time of offer:
- Age
- Sex
- Height
- Blood group
- Negative SARS-CoV-2 swabs
- Serological result for HIV, HBV, HCV, or the time that this information will be available
HLA tissue typing should be subsequently available after the transplant if it is not available at the time of retrieval.
The following information should be provided to determine the likelihood of death occurring:
- Primary diagnosis and past medical history
- The use and dose of inotropes
- Presence of a gag reflex
- Presence of a cough reflex
- Respiratory rate when disconnected from the ventilator
- The fraction of inspired oxygen (FiO2)
- Arterial oxygen saturation and pH
- Ventilation mode
- Planned time of withdrawal of treatment
- Planned mode of withdrawal of treatment – i.e., extubation, reduction of inotropes
The following should be available, but with varying detail:
- Arterial blood gases, ideally on 100% and PEEP 5 cmH2 Not all intensive care units (ICUs) will report this, but the ABG and FiO2 of the last blood gas, within less than 12 hours of procurement, should be available.
- Chest X-ray within 24 hours of procurement. The chest X-ray report should be available (based on which some of the contraindications may be identified, i.e., extensive bilateral lower lobe consolidation).
- The consensus document states, “bronchoscopy to assess the potential for lung donation may be appropriate if it does not cause the patient distress”. This needs specific discussion with the patient’s family. Bronchoscopy should be requested as a routine, although there is no obligation for the ICU to carry it out. There is no need for gram staining of tracheal or central airway secretions.
13.3 Organ Preservation
The lungs are antegradely flushed with reconstituted Perfadex Plus ® to which prostacyclin is added, the first litre at room temperature, the rest at 4°C. A volume of 50-60 mL/kg is given. The lungs are gently ventilated to maximise distribution of the perfusate.
After the antegrade dose, 250 mL will be given down each pulmonary vein as a final retrograde flush. This can be given in situ or on the back table. An additional dose of 250 mL can be considered if clots are visualised in the initial flush.
The UK national protocol for procurement of lungs in DCD donors has been recently updated and should be referred to (2).
In DCD donors with abdominal normothermic regional perfusion (NRP), the goals are to procure the lungs as safely as possible in a way that the perfusion to the abdominal organs is also preserved. When the heart and lungs are being procured the heart is explanted first, followed by antegrade perfusion to the lungs and retrograde perfusion through the pulmonary veins. The remaining dissection of the lungs is commenced after 30 minutes of abdominal NRP, which is crucial for liver recovery and to avoid damage to any blood vessels in the mediastinum. This time is utilised for bronchoscopy and further assessment of the lungs in the form of inspection and palpation after appropriate ventilation to examine all the segments, especially those areas which might have atelectasis during the agonal phase.
If only lungs are being procured, the antegrade perfusion is delivered after clamping the pulmonary artery proximally. After 30 minutes of abdominal NRP the dissection is commenced and the heart and lungs are procured en bloc. Retrograde perfusion is then performed on the back table after the heart is excised. The heart is returned to the chest and careful haemostasis is performed in the chest as excess bleeding may result in an unusable liver, kidney or pancreas.
For lungs destined for EVLP, an adequate portion of the main pulmonary artery (PA), left atrial cuff and particularly at least 4 cm of the trachea will be taken by the procurement surgeon. A piece of the aorta will be required to extend a deficient main PA (divided in close proximity to the bifurcation) to allow for successful cannulation and bilateral perfusion.
13.4 Organ Quality Assessment
Testing graft function of warm perfused organs prior to transplantation is considered the ‘gold standard’. The test is performed by ventilating the lungs and perfusing them with Steen solution +/- added red blood cells. The ability of the ventilated lung to oxygenate the perfusate is assessed together with lung compliance, airway resistance and tidal volume via the ventilator.
13.5 Recipient Selection
There are now internationally used acceptance criteria for listing lung transplant patients (3). All patients on the lung transplant waiting list have the potential to receive DCD lungs. Most centres in the UK have included ‘DCD donor’ in their list of categories for which specific consent is obtained, but do not regard it as implying any additional risk. The allocation of lungs is as per the NHSBT guidelines for DBD lung donation. https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/24243/pol230-12.pdf
13.6 Immunosuppression
There is currently no evidence to suggest that there should be any difference in the immunosuppression protocol for DCD versus DBD lung transplantation.
13.7 Outcomes
Since the first published results in the modern era (4), there have been institutional reports of lung transplantation after procurement from Category III donors from a number of centres around the world. These include series from Melbourne (4), Toronto (5), St Louis (6), and Leuven (7). In general, outcomes were at least as good as contemporaneous transplants from DBD donors, although there may be an element of publication bias. Comparisons made include 30-day and one-year mortality, and in some of the papers, the incidence of primary graft dysfunction, acute rejection and early obliterative bronchiolitis. In no series was there higher morbidity or mortality (8).
The use of EVLP after DCD lung donation has been recently reported in both abstracts (9) and in one landmark paper (10), again with excellent results. A further update has confirmed excellent outcomes with this treatment (11).
One of the earliest DCD reports was the case report of Steen describing EVLP for the evaluation of lungs from an uncontrolled DCD (uDCD) donor, and a subsequent successful transplant (12). The Madrid group has subsequently reported medium- and long-term results in 29 uDCD donors (13). They found that there was a higher incidence of primary graft dysfunction resulting in higher early mortality. Thereafter uDCD donation was not taken up in many centres. A recent review has suggested EVLP in this subset of donors to increase organ procurement from this group to improve the donor pool (14).
Van Raemdonck et al recently reported follow-up data from the ISHLT DCD Registry (15). The report included 11,516 lung transplants, of which 1,090 (9.5%) were DCD lung transplants. They concluded in this registry study that five-year follow-up demonstrated similar favourable long-term survival in DBD and DCD transplants.
Palleschi et al reported a systematic review and meta-analysis of nine studies regarding controlled DCD including 2973 patients (403 received a DCD graft and 2570 had DBD) (16). The meta-analysis showed no significant difference between recipients after DCD or DBD regarding one-year survival, primary graft dysfunction and one-year freedom from chronic lung allograft dysfunction.
In conclusion, the results of transplantation with lungs from Category III DCD donors are excellent.
References
- Donation after Circulatory Death: A Consensus Statement from the British Transplantation Society and Intensive Care Society. Available at bts.org.uk.
- UK National Protocol for direct retrieval and perfusion (DRP) of DCD Hearts and Lungs with or without abdominal NRP (A-NRP) to Ex-situ Normothermic perfusion. Available at https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/25288/01112021uk-national-protocol-for-direct-retrieval-of-dcd-hearts.pdf.
- LeardLE, Holm AM, Valapour M et al. Consensus document for the selection of lung transplant candidates: An update from the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2021 Nov;40(11):1349-79.
- Snell GI, Levvey BJ, Oto T, et al. Early lung transplantation success utilizing controlled donation after cardiac death donors. Am J Transplantation 2008;8:1282-9.
- Cypel M, Sato M, Yildirim E, et al. Initial experience with lung donation after cardiocirculatory death in Canada. J Heart Lung Transplant 2009;28:753-8.
- Puri V, Scavuzzo M, Guthrie T, et al. Lung transplantation and donation after cardiac death: a single center experience. Ann Thorac Surgery 2009;88:1609-14.
- De Vleeschauwer SI, Wauters S, Dupont LJ, et al. Medium-term outcome after lung transplantation is comparable between brain-dead and cardiac-dead donors. J Heart Lung Transplantation 2011;30:975-81.
- Wigfield CH, Love RB. Donation after cardiac death lung transplantation outcomes. Curr Opin Organ Transplant 2011;16:462-8.
- Cypel M, Yeung JC, Keshavjee S. Novel approaches to expanding the lung donor pool: donation after cardiac death and ex vivo conditioning. Clin Chest Medicine 2011;32:233-44.
- Cypel M, Yeung JC, Liu M, et al., Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med 2011;364:1431-40.
- Cypel M, Yeung JC, Machuca T, et al. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J Thorac Cardiovasc Surg 2012;144:1200-6.
- Steen S, Sjöberg T, Pierre L, et al. Transplantation of lungs from a non-heart-beating donor. Lancet 2001;357:825-9.
- Gomez-de-Antonio D, Campo-Cañaveral JL, Crowley S et al. Clinical lung transplantation from uncontrolled non-heart-beating donors revisited. J Heart Lung Transplant 2012 Apr;31(4):349-53.
- EganTM, Haithcock BE , Lobo J et al. Donation after circulatory death donors in lung transplantation. J Thorac Dis 2021 Nov;13(11):6536-49.
- Van Raemdonck D, Keshavjee S, Levvey B et al. Donation after circulatory death in lung transplantation-five-year follow-up from ISHLT Registry. J Heart Lung Transplant 2019 Dec;38(12):1235-45.
- Palleschi A, Rosso A, MussoV et al. Lung transplantation from donation after controlled cardiocirculatory death. Systematic review and meta-analysis. Transplant Rev (Orlando); 2020 Jan;34(1):100513.
14. Heart
We recommend that:
- All patients on the heart transplant waiting list have the potential to receive DCD hearts. (1C)
We suggest that:
- The donor selection criteria for heart DCD transplantation should follow the current guidance from NHSBT. (2B)
14.1 Introduction
Heart transplantation following DCD donation was introduced to clinical practice in 2014. In the last 7 years, the practice has flourished in the UK, Europe, USA and Australia with results comparable to DBD donation (1,2).
14.2 Donor Selection
The donor selection criteria for heart DCD should be similar to those for DBD except the age of the donor is currently 50 years or less. Organ donation is expensive, so the likelihood of respiratory and subsequent cardiac arrest is made with the following criteria: primary diagnosis and past medical history, the dose of inotropes, presence of a gag reflex, presence of a cough reflex, respiratory rate when disconnected from the ventilator, FiO2, presence and quantity of PEEP, arterial blood gas result, mode and timing of withdrawal of life-sustaining treatment.
Donor inclusion criteria:
- Controlled DCD (Maastricht Category III and IV)
- Age ≤50 years
- Weight ≥40 kg (if suitable paediatric donors, refer to DCD paediatric protocol for <30kg donors)
- Consent/authorisation obtained from next of kin/ organ donor register
Donor exclusion criteria (*relative contraindication)
- Previous cardiac surgery or midline sternotomy
- Valvular heart disease
- Congenital heart disease*
- Significant coronary artery disease
- Chronic atrial fibrillation*
- Insulin-dependent diabetes*
- HIV+
- Current intravenous drug abuse
- Tumour with high risk of transmission according to guidelines of the Advisory Committee on the Safety of Blood, Tissues and Organs (SaBTO)
14.3 Donor Warm Ischaemic Times
The following times are helpful in assessing the magnitude of ischaemic insult to the donor heart:
- Withdrawal of life-sustaining treatment (WLST) to onset of functional warm ischaemia (FWIT: at systolic blood pressure 50 mmHg, noting, however, that the heart is a diastolic perfused organ).
- Time to loss of pulsatility on peripheral arterial monitoring (onset of circulatory arrest).
- Time of confirmation of death (five minutes after the above. Note: to await a flat ECG adds a further 8 minutes of myocardial ischaemia).
- The time of cold cardioplegia delivery (this marks the end of FWIT).
- The onset of heart perfusion with oxygenated blood-based perfusate.
- The total time of ex situ machine perfusion (TESMP) is also required.
14.4 Donor Organ Retrieval Specifics
14.4.2 Required Information at the Donor Hospital
Probably the most important action is the multi-team huddle on the arrival of all teams, at the donor hospital. This is an opportunity for all members of the retrieval teams to propose, assemble and understand the sequence of steps required to ensure safe, effective multi-organ retrieval. Particularly important is the detail addressing:
- Withdrawal of 1.5 L of donor blood following intravenous heparin 25,000 IU into both the right atrium and pulmonary artery. This takes no longer than 1 minute before the delivery of any organ preservation solution by either the cardio-thoracic or abdominal teams.
- The plan to use thoraco-abdominal NRP (thoraco-abdominal normothermic regional perfusion), abdominalNRP (abdominal normothermic regional perfusion) or DPP (direct procurement and ex situ machine perfusion).
- The plan by the cardio-thoracic team to ensure against significant blood loss if the heart is to be retrieved by DPP in a case of abdominal NRP (particularly over superior and inferior vena-caval and azygous vein [with attention to the presence of a left-sided SVC with a hemi-azygous to coronary sinus connection] occlusion).
- If thoraco-abdominal NRP (or in situ machine perfusion: ISMP) is to be used, the abdominal team usually cannulate both a large vein and artery within the abdomen or groin, with 50,000 IU heparin in the prime solution. The cardio-thoracic team will cross-clamp the descending thoracic aorta within the chest (usually through the posterior pericardium). Before releasing the descending thoracic aortic clamp, the cardio-thoracicteam will have occluded the arch vessels (stapling) and excluded the presence of an occluded and anomalous right subclavian artery. Arrangement will be made to drain the occluded arch arteries by drain insertion beyond the staple line with any retrieved blood directed to the NRP reservoir.
14.5 Organ Preservation
ESMP perfusate
A minimum of 1.2 L donor blood is collected with a raised table in head down position. It is crucial to ensure that no preservation solution is given until donor blood is drained, and no vasoconstrictor bolus is given at this stage.
- Drainage of donor blood follows the injection of 25,000 IU of heparin into each of the right atrium and pulmonary artery.
- This should take no more than 60 seconds through a right atrial two-stage venous cannula connected to a blood collection bag with the OCS priming solution containing 60,000 IU of heparin.
- During donor blood collection the cardiothoracic surgeon will clamp the descending aorta above the diaphragm, as low as possible.
- The cardiothoracic surgeon will announce this clamp is in place and the time will be recorded on the National DCD Heart Passport.
- A clamp is then placed across the distal ascending aorta and a DLP cannula is inserted into the ascending aorta for cold cardioplegia delivery.
- The heart is excised in the standard fashion for heart retrieval.
- If the lungs are to be retrieved a reasonable cuff of pulmonary veins and reasonable length of left and right pulmonary arteries are ensured. The local hospital anaesthetist or NORS team donor care practitioner will have to reintubate the donor trachea during sternotomy. Care must be taken to leave the posterior wall of PA when removing the heart. After removal of the heart, antegrade pneumoplegia is delivered through both left and right pulmonary cannulae. This is followed by retrograde pneumoplegia delivered through cannulated pulmonary
14.6 Ex situ Machine Perfusion (ESMP)
14.6.1 Preparation of the DCD Heart Prior to Ex situ Perfusion
- The heart is immediately placed into a basin of ice-cold sterile saline solution.
- Swift dissection is made to free the aorta from the pulmonary artery allowing for placing an appropriately sized ESMP connector using a cable tie.
- The heart is placed onto the primed OCS ESMP device and de-aired.
- An LV vent is secured through the left atrium into the organ chamber to ensure against left ventricular distention.
- Following reperfusion of the heart the aorta is secured further by three Teflon pledgeted aortic stitches into the distal aorta to reduce the risk of disconnection during travel to the recipient hospital.
- Right ventricular pacing wires are placed in case of the need for pacing.
14.6.2 Perfusion Parameters During Transport
Commence OCS perfusion of donor heart aiming for:
- Mean AOP 55-70 mmHg
- Aortic flow of 550-1100 mL/min (aortic flow = coronary flow plus de-airing line flow [order of 50mL/min])
- Coronary flow 500-750 mL/min (see above)
- Heart rate 50-90 BPM with V-pacing
Once heart rhythm and perfusion are stable consider synchronising perfusion with rhythm after discussion with the implanting team.
14.6.3 Perfusate Biochemistry
Acquire simultaneous AV blood samples every 30 minutes or more frequently if needed. In correction of perfusate biochemistry, the perfusate volume is noted to be of the order of 2 L and suggested targets are:
- Hct >15%
- Calcium 1.0-1.3 mmol/L
- Bicarbonate 22-29 mmol/L
- pH – 7.3-7.45
- Glucose 5.0-8.0 mmol/L
14.6.4 During Transport
Ensure to travel with a safety ice box and roadside bag which will include: ice, cardioplegia, giving set and pressure bag, and 8 litres of cold saline.
- This offers cardioplegia and cold storage should the heart become detached from ESMP.
- Roadside bag – sterile instruments, sterile gloves of different sizes, sterile gowns, three packing bags for the heart.
14.7 Organ Quality Assessment
This is made up of assessment:
- Prior to WLST by trans-oesophageal or thoracic echocardiography paying attention to the left and right ventricular contraction, wall thickness and valve function.
- Just prior to ESMP the heart is weighed (expressed as initial wet weight).
- During surgical heart retrieval by feeling for coronary artery disease.
- During ESMP after commencing heart perfusion. Contractility, rhythm and half-hourly arterial and venous blood gas sampling are recorded during the time of transport to the recipient centre.
- There is no evidence that perfusate lactate measurement is of use in describing the quality of the donor heart (3).
14.8 Recipient Selection
All recipients on the heart transplant waiting list are offered the opportunity to receive a DCD donor heart in addition to waiting for a DBD heart. It is important to obtain written informed consent from the potential recipient noting awareness of DCD heart donation and the use, where appropriate, of thoraco-abdominal NRP.Implantation of the cold arrested, prepared, DCD donor heart is made ideally with myocardial reperfusion using cold cardioplegic blood-based perfusate ante- (aortic root) or retro-grade via the coronary sinus.
14.9 Immunosuppression
There is currently no evidence indicating that there should be a difference in the immunosuppression protocol for DCD when compared to DBD heart transplantation.
14.10 Outcomes
Outcomes following heart transplantation from DCD donors are comparable to heart transplantation from DBD donors (1,2).
14.10.1 Recording of outcomes of DCD heart donation
The following outcomes should be recorded after DCD heart transplantation:
- The wet weight following ESMP prior to implantation.
- At the time of weaning the transplanted heart from cardio-pulmonary bypass, record the need for pharmacological and or machine support. The duration of this support and any changes made to it should also be recorded.
- The time on the ventilator following surgery and the duration of ITU stay.
- The presence of acute rejection and need for treatment of this.
- The 30-day, 90-day and one-year mortality.
14.11 Paediatric DCD Heart Transplantation
Heart transplantation has been successfully performed in paediatric patients (4,5) and began in the early part of this millennium supported by a strategy of minimising the warm ischaemic time. Recent reports describe successful DCD heart transplantation in children over 38 kg. This restriction reflects the limits of ESMP made possible by the machines currently available.
References
- Messer S,Cernic S, Page A, et al. A 5-year single-center early experience of heart transplantation from donation after circulatory determined death donors. J Heart Lung Transplant 2020;39:1463−75.
- Dhital K, Ludhani P,Scheuer S , et al. DCD donations and outcomes of heart transplantation: the Australian experience. Indian J of Thorac Cardiovasc Surg 2020 August 36 (Suppl 2):S224-32.
- Cernic S, Page A, Messer A et al.Lactate during ex-situ heart perfusion does not predict the requirement for mechanical circulatory support following donation after circulatory death (DCD) heart transplants. J Heart Lung Transplant 2022;41(9):1294-1302.
- Boucek MM, Mashburn C, Dunn SM et al. Pediatric heart transplantation after declaration of cardiocirculatory death. N Engl J Med 2008;359(7):709-14.
- Laurence C, Nachum E, Henwood S et al. Pediatric heart transplantation following donation after circulatory death, distant procurement, and ex-situ perfusion. J Heart Lung Transplant 2022;41(8):1104-13.